Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die
- PMID: 15210730
- PMCID: PMC2172390
- DOI: 10.1083/jcb.200312031
Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die
Abstract
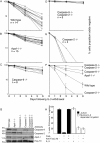
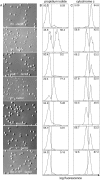
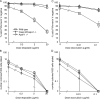
Apoptosis after growth factor withdrawal or drug treatment is associated with mitochondrial cytochrome c release and activation of Apaf-1 and caspase-9. To determine whether loss of Apaf-1, caspase-2, and caspase-9 prevented death of factor-starved cells, allowing them to proliferate when growth factor was returned, we generated IL-3-dependent myeloid lines from gene-deleted mice. Long after growth factor removal, cells lacking Apaf-1, caspase-9 or both caspase-9 and caspase-2 appeared healthy, retained intact plasma membranes, and did not expose phosphatidylserine. However, release of cytochrome c still occurred, and they failed to form clones when IL-3 was restored. Cells lacking caspase-2 alone had no survival advantage. Therefore, Apaf-1, caspase-2, and caspase-9 are not required for programmed cell death of factor-dependent cells, but merely affect its rate. In contrast, transfection with Bcl-2 provided long-term, clonogenic protection, and could act independently of the apoptosome. Unlike expression of Bcl-2, loss of Apaf-1, caspase-2, or caspase-9 would therefore be unlikely to enhance the survival of cancer cells.
Figures



References
-
- Cecconi, F., G. Alvarezbolado, B.I. Meyer, K.A. Roth, and P. Gruss. 1998. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 94:727–737. - PubMed
-
- Chinnaiyan, A.M., K. Orourke, B.R. Lane, and V.M. Dixit. 1997. Interaction of CED-4 with CED-3 and CED-9 - a molecular framework for cell death. Science. 275:1122–1126. - PubMed
-
- Hakem, R., A. Hakem, G.S. Duncan, J.T. Henderson, M. Woo, M.S. Soengas, A. Elia, J.L. Delapompa, D. Kagi, W. Khoo, et al. 1998. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 94:339–352. - PubMed

