The LIM domain protein UNC-95 is required for the assembly of muscle attachment structures and is regulated by the RING finger protein RNF-5 in C. elegans
- PMID: 15210732
- PMCID: PMC2172400
- DOI: 10.1083/jcb.200401133
The LIM domain protein UNC-95 is required for the assembly of muscle attachment structures and is regulated by the RING finger protein RNF-5 in C. elegans
Abstract
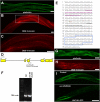
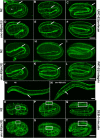
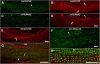
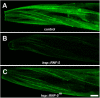
Here, we describe a new muscle LIM domain protein, UNC-95, and identify it as a novel target for the RING finger protein RNF-5 in the Caenorhabditis elegans body wall muscle. unc-95(su33) animals have disorganized muscle actin and myosin-containing filaments as a result of a failure to assemble normal muscle adhesion structures. UNC-95 is active downstream of PAT-3/beta-integrin in the assembly pathways of the muscle dense body and M-line attachments, and upstream of DEB-1/vinculin in the dense body assembly pathway. The translational UNC-95::GFP fusion construct is expressed in dense bodies, M-lines, and muscle-muscle cell boundaries as well as in muscle cell bodies. UNC-95 is partially colocalized with RNF-5 in muscle dense bodies and its expression and localization are regulated by RNF-5. rnf-5(RNAi) or a RING domain deleted mutant, rnf-5(tm794), exhibit structural defects of the muscle attachment sites. Together, our data demonstrate that UNC-95 constitutes an essential component of muscle adhesion sites that is regulated by RNF-5.
Figures



References
-
- Bach, I. 2000. The LIM domain: regulation by association. Mech. Dev. 91:5–17. - PubMed
-
- Dawid, I.B., J.J. Breen, and R. Toyama. 1998. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 14:156–162. - PubMed
-
- Didier, C., L. Broday, A. Bhoumik, S. Israeli, S. Takahashi, K. Nakayama, S.M. Thomas, C.E. Turner, S. Henderson, H. Sabe, and Z. Ronai. 2003. RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization. Mol. Cell. Biol. 23:5331–5345. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

