Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms
- PMID: 15210734
- PMCID: PMC2172406
- DOI: 10.1083/jcb.200403174
Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms
Abstract
Melanoma chondroitin sulfate proteoglycan (MCSP) is an early cell surface melanoma progression marker implicated in stimulating tumor cell proliferation, migration, and invasion. Focal adhesion kinase (FAK) plays a pivotal role in integrating growth factor and adhesion-related signaling pathways, facilitating cell spreading and migration. Extracellular signal-regulated kinase (ERK) 1 and 2, implicated in tumor growth and survival, has also been linked to clinical melanoma progression. We have cloned the MCSP core protein and expressed it in the MCSP-negative melanoma cell line WM1552C. Expression of MCSP enhances integrin-mediated cell spreading, FAK phosphorylation, and activation of ERK1/2. MCSP transfectants exhibit extensive MCSP-rich microspikes on adherent cells, where it also colocalizes with alpha4 integrin. Enhanced activation of FAK and ERK1/2 by MCSP appears to involve independent mechanisms because inhibition of FAK activation had no effect on ERK1/2 phosphorylation. These results indicate that MCSP may facilitate primary melanoma progression by enhancing the activation of key signaling pathways important for tumor invasion and growth.
Figures


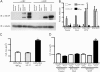


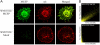
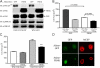
References
-
- Aplin, A.E., and R.L. Juliano. 1999. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J. Cell Sci. 112:695–706. - PubMed
-
- Barberis, L., K.K. Wary, G. Fiucci, F. Liu, E. Hirsch, M. Brancaccio, F. Altruda, G. Tarone, and F.G. Giancotti. 2000. Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J. Biol. Chem. 275:36532–36540. - PubMed
-
- Burg, M.A., K.A. Grako, and W.B. Stallcup. 1998. Expression of the NG2 proteoglycan enhances the growth and metastatic properties of melanoma cells. J. Cell. Physiol. 177:299–312. - PubMed
-
- Burridge, K., and M. Chrzanowska-Wodnicka. 1996. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12:463–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

