T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin
- PMID: 15210937
- PMCID: PMC478568
- DOI: 10.1073/pnas.0403382101
T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin
Abstract
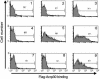
Acrp30/adiponectin is reduced in the serum of obese and diabetic individuals, and the genetic locus of adiponectin is linked to the metabolic syndrome. Recombinant adiponectin, administered to diet-induced obese mice, induced weight loss and improved insulin sensitivity. In muscle and liver, adiponectin stimulates AMP-activated protein kinase activation and fatty acid oxidation. To expression-clone molecules capable of binding adiponectin, we transduced a C2C12 myoblast cDNA retroviral expression library into Ba/F3 cells and panned infected cells on recombinant adiponectin linked to magnetic beads. We identified T-cadherin as a receptor for the hexameric and high-molecular-weight species of adiponectin but not for the trimeric or globular species. Only eukaryotically expressed adiponectin bound to T-cadherin, implying that posttranslational modifications of adiponectin are critical for binding. An adiponectin mutant lacking a conserved N-terminal cysteine residue required for formation of hexamer and high-molecular-weight species did not bind T-cadherin in coimmunoprecipitation studies. Although lacking known cellular functions, T-cadherin is expressed in endothelial and smooth muscle cells, where it is positioned to interact with adiponectin. Because T-cadherin is a glycosylphosphatidylinositol-anchored extracellular protein, it may act as a coreceptor for an as-yet-unidentified signaling receptor through which adiponectin transmits metabolic signals.
Figures

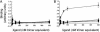

References
-
- Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. (1995) J. Biol. Chem. 270, 26746–26749. - PubMed
-
- Tsao, T. S., Tomas, E., Murrey, H. E., Hug, C., Lee, D. H., Ruderman, N. B., Heuser, J. E. & Lodish, H. F. (2003) J. Biol. Chem. 278, 50810–50817. - PubMed
-
- Hara, K., Boutin, P., Mori, Y., Tobe, K., Dina, C., Yasuda, K., Yamauchi, T., Otabe, S., Okada, T., Eto, K., et al. (2002) Diabetes 51, 536–540. - PubMed
-
- Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K., Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., et al. (2001) Nat. Med. 7, 941–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

