Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines
- PMID: 15210954
- PMCID: PMC470709
- DOI: 10.1073/pnas.0403549101
Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines
Abstract
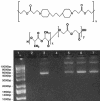
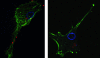
Current nonviral genetic vaccine systems are less effective than viral vaccines, particularly in cancer systems where epitopes can be weakly immunogenic and antigen-presenting cell processing and presentation to T cells is down-regulated. A promising nonviral delivery method for genetic vaccines involves microencapsulation of antigen-encoding DNA, because such particles protect plasmid payloads and target them to phagocytic antigen-presenting cells. However, conventional microparticle formulations composed of poly lactic-co-glycolic acid take too long to release encapsulated payload and fail to induce high levels of target gene expression. Here, we describe a microparticle-based DNA delivery system composed of a degradable, pH-sensitive poly-beta amino ester and poly lactic-co-glycolic acid. These formulations generate an increase of 3-5 orders of magnitude in transfection efficiency and are potent activators of dendritic cells in vitro. When used as vaccines in vivo, these microparticle formulations, unlike conventional formulations, induce antigen-specific rejection of transplanted syngenic tumor cells.
Figures
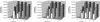

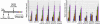
References
-
- Gurunathan, S., Klinman, D. M. & Seder, R. A. (2000) Annu. Rev. Immunol. 18, 927-974. - PubMed
-
- McKenzie, B. S., Corbett, A. J., Brady, J. L., Dyer, C. M., Strugnell, R. A., Kent, S. J., Kramer, D. R., Boyle, J. S. & Lew, A. M. (2001) Immunol. Res. 24, 225-244. - PubMed
-
- Pardoll, D. M. (2002) Nat. Rev. Immunol. 2, 227-238. - PubMed
-
- McKeever, U., Barman, S., Hao, T., Chambers, P., Song, S., Lunsford, L., Hsu, Y. Y., Roy, K. & Hedley, M. L. (2002) Vaccine 20, 1524-1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

