A molecular link between SR protein dephosphorylation and mRNA export
- PMID: 15210956
- PMCID: PMC470732
- DOI: 10.1073/pnas.0403533101
A molecular link between SR protein dephosphorylation and mRNA export
Abstract
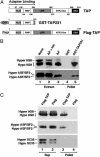
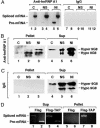
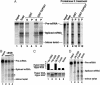
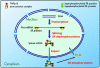
In metazoans, multiple RNA-binding proteins, including the shuttling serine/arginine-rich (SR)-splicing factors, function as adapters for mRNA nuclear export by interacting with the export receptor TAP/nuclear export factor 1 (NXF1). Yet, it is unclear how interactions between adapters and TAP are regulated. Here, we demonstrate that the SR proteins 9G8 and ASF/SF2 exhibit higher affinity for TAP/NXF1 when hypophosphorylated. 9G8 is recruited to the pre-mRNA in a hyperphosphorylated form but becomes hypophosphorylated during splicing both in vivo and in vitro. TAP preferentially binds spliced mRNA-protein complexes compared with pre-mRNA-protein complexes. Thus, the phosphorylation state of the SR protein adapters may underlie the selectivity of TAP-mediated export of spliced mRNA.
Figures
References
-
- Dreyfuss, G., Kim, V. N. & Kataoka, N. (2002) Nat. Rev. Mol. Cell Biol. 3, 195-205. - PubMed
-
- Stutz, F. & Izaurralde, E. (2003) Trends Cell Biol. 13, 319-327. - PubMed
-
- Reed, R. & Hurt, E. (2002) Cell 108, 523-531. - PubMed
-
- Straber, K., Masuda, S., Mason, P., Pfannstiel, J., Oppizzi, M., Rodriguez-Navarro, S., Rondon, A. G., Aguilera, A., Struhl, K., Reed, R. & Hurt, E. (2002) Nature 28, 1-4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

