The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging
- PMID: 15210960
- PMCID: PMC470762
- DOI: 10.1073/pnas.0403495101
The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging
Abstract
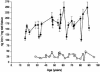
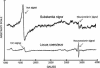
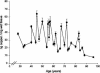
In this study, a comparative analysis of metal-related neuronal vulnerability was performed in two brainstem nuclei, the locus coeruleus (LC) and substantia nigra (SN), known targets of the etiological noxae in Parkinson's disease and related disorders. LC and SN pars compacta neurons both degenerate in Parkinson's disease and other Parkinsonisms; however, LC neurons are comparatively less affected and with a variable degree of involvement. In this study, iron, copper, and their major molecular forms like ferritins, ceruloplasmin, neuromelanin (NM), manganese-superoxide dismutase (SOD), and copper/zinc-SOD were measured in LC and SN of normal subjects at different ages. Iron content in LC was much lower than that in SN, and the ratio heavy-chain ferritin/iron in LC was higher than in the SN. The NM concentration was similar in LC and SN, but the iron content in NM of LC was much lower than SN. In both regions, heavy- and light-chain ferritins were present only in glia and were not detectable in neurons. These data suggest that in LC neurons, the iron mobilization and toxicity is lower than that in SN and is efficiently buffered by NM. The bigger damage occurring in SN could be related to the higher content of iron. Ferritins accomplish the same function of buffering iron in glial cells. Ceruloplasmin levels were similar in LC and SN, but copper was higher in LC. However, the copper content in NM of LC was higher than that of SN, indicating a higher copper mobilization in LC neurons. Manganese-SOD and copper/zinc-SOD had similar age trend in LC and SN. These results may explain at least one of the reasons underlying lower vulnerability of LC compared to SN in Parkinsonian syndromes.
Figures
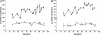
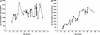


References
-
- Foote, S. L., Freedman, R. & Oliver, A. P. (1975) Brain Res. 86, 229-242. - PubMed
-
- Arnsten, A. F. & Goldman-Rakic, P. S. (1987) J. Neural Transm. 24, 317-324. - PubMed
-
- Mason, S. T. & Fibiger, H. C. (1979) J. Comp. Neurol. 187, 703-724. - PubMed
-
- Nygren, L. G. & Olson, L. (1977) Brain Res. 132, 85-93. - PubMed
-
- German, D. C., Manaye, K. F., White, C. L. III, Woodward, D. J., McIntire, D. D., Smith, W. K., Kalaria, R. N. & Mann, D. M. (1992) Ann. Neurol. 32, 667-676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

