Cells release prions in association with exosomes
- PMID: 15210972
- PMCID: PMC470735
- DOI: 10.1073/pnas.0308413101
Cells release prions in association with exosomes
Abstract
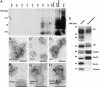
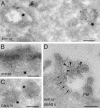
Prion diseases are infectious neurodegenerative disorders linked to the accumulation in the central nervous system of the abnormally folded prion protein (PrP) scrapie (PrPsc), which is thought to be the infectious agent. Once present, PrPsc catalyzes the conversion of naturally occurring cellular PrP (PrPc) to PrPsc. Prion infection is usually initiated in peripheral organs, but the mechanisms involved in infectious spread to the brain are unclear. We found that both PrPc and PrPsc were actively released into the extracellular environment by PrP-expressing cells before and after infection with sheep prions, respectively. Based on Western blot with specific markers, MS, and morphological analysis, our data revealed that PrPc and PrPsc in the medium are associated with exosomes, membranous vesicles that are secreted upon fusion of multivesicular endosomes with the plasma membrane. Furthermore, we found that exosomes bearing PrPsc are infectious. Our data suggest that exosomes may contribute to intercellular membrane exchange and the spread of prions throughout the organism.
Figures



Similar articles
-
Exosomes: a bubble ride for prions?Traffic. 2005 Jan;6(1):10-7. doi: 10.1111/j.1600-0854.2004.00247.x. Traffic. 2005. PMID: 15569241 Review.
-
Prions and exosomes: from PrPc trafficking to PrPsc propagation.Blood Cells Mol Dis. 2005 Sep-Oct;35(2):143-8. doi: 10.1016/j.bcmd.2005.06.013. Blood Cells Mol Dis. 2005. PMID: 16099696 Review.
-
Generation of cell lines propagating infectious prions and the isolation and characterization of cell-derived exosomes.Methods Mol Biol. 2008;459:69-82. doi: 10.1007/978-1-59745-234-2_5. Methods Mol Biol. 2008. PMID: 18576148
-
Isolation of Exosomes and Microvesicles from Cell Culture Systems to Study Prion Transmission.Methods Mol Biol. 2017;1545:153-176. doi: 10.1007/978-1-4939-6728-5_11. Methods Mol Biol. 2017. PMID: 27943213
-
Genetic and infectious prion diseases.Arch Neurol. 1993 Nov;50(11):1129-53. doi: 10.1001/archneur.1993.00540110011002. Arch Neurol. 1993. PMID: 8105771 Review.
Cited by
-
Extracellular Vesicles in Neurological Disorders.Subcell Biochem. 2021;97:411-436. doi: 10.1007/978-3-030-67171-6_16. Subcell Biochem. 2021. PMID: 33779926
-
Ultrastructural changes in the progress of natural Scrapie regardless fixation protocol.Histochem Cell Biol. 2015 Jul;144(1):77-85. doi: 10.1007/s00418-015-1314-6. Epub 2015 Feb 28. Histochem Cell Biol. 2015. PMID: 25724812
-
CSF prion protein concentration and cognition in patients with Alzheimer disease.Prion. 2013 May-Jun;7(3):229-34. doi: 10.4161/pri.23904. Epub 2013 Feb 13. Prion. 2013. PMID: 23406922 Free PMC article.
-
Progress and gaps of extracellular vesicle-mediated intercellular cargo transfer in the central nervous system.Commun Biol. 2022 Nov 11;5(1):1223. doi: 10.1038/s42003-022-04050-z. Commun Biol. 2022. PMID: 36369335 Free PMC article. Review.
-
Separate mechanisms act concurrently to shed and release the prion protein from the cell.Prion. 2012 Nov-Dec;6(5):498-509. doi: 10.4161/pri.22588. Epub 2012 Oct 23. Prion. 2012. PMID: 23093798 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

