Unified synthesis of caged Garcinia natural products based on a site-selective Claisen/Diels-Alder/Claisen rearrangement
- PMID: 15210986
- PMCID: PMC514429
- DOI: 10.1073/pnas.0401932101
Unified synthesis of caged Garcinia natural products based on a site-selective Claisen/Diels-Alder/Claisen rearrangement
Abstract
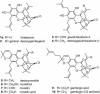
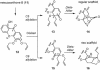
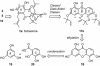
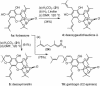
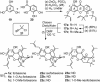
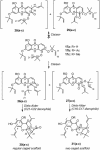
A unified synthetic strategy toward caged Garcinia natural products has been designed and implemented. Central to the strategy is a tandem Claisen/Diels-Alder/Claisen rearrangement of a suitably substituted xanthone precursor to form forbesione (1a). Serving as a template, forbesione is then used to deliver representative members of this family, including desoxygaudichaudione A (4), desoxymorellin (5), and gambogin (10). Studies on the timing of this reaction cascade suggest that the C-ring Claisen/Diels-Alder rearrangement proceeds initially and is followed by the A-ring Claisen reaction. The electronic and steric effects that govern the outcome of this cascade are presented.
Figures

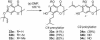
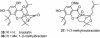
References
-
- Ollis, W. D., Redman, B. T., Sutherland, I. O. & Jewers, K. (1969) J. Chem. Soc. Chem. Commun., 879-880.
-
- Kumar, P. & Baslas, R. K. (1980) Herba Hung. 19, 81-91.
-
- Thoison, O., Fahy, J., Dumontet, V., Chiaroni, A., Riche, C., Tri, M. V. & Sevenet, T. (2000) J. Nat. Prod. 63, 441-446. - PubMed
-
- Gruenwald, J., Brendler, T. & Jaenicke, C., eds. (2000) PDR for Herbal Medicines (Medical Economics, Montvale, NJ), 2nd Ed., pp. 325-326.
-
- Kartha, G., Ramachandran, G. N., Bhat, H. B., Nair, P. M., Raghavan, V. K. V. & Venkataraman, K. (1963) Tetrahedron Lett. 4, 459-462.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

