Mucosal vaccination against serogroup B meningococci: induction of bactericidal antibodies and cellular immunity following intranasal immunization with NadA of Neisseria meningitidis and mutants of Escherichia coli heat-labile enterotoxin
- PMID: 15213150
- PMCID: PMC427466
- DOI: 10.1128/IAI.72.7.4052-4060.2004
Mucosal vaccination against serogroup B meningococci: induction of bactericidal antibodies and cellular immunity following intranasal immunization with NadA of Neisseria meningitidis and mutants of Escherichia coli heat-labile enterotoxin
Abstract
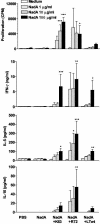
Conjugated polysaccharide vaccines protect against serogroup C meningococci. However, this approach cannot be applied to serogroup B, which is still a major cause of meningitis. We evaluated the immunogenicity of three surface-exposed proteins from serogroup B Neisseria meningitidis (App, NhhA, and NadA) identified during whole-genome sequencing. Mice were immunized intranasally with individual proteins in the presence of wild-type Escherichia coli heat-labile enterotoxin (LTwt), LTR72, a partially inactivated mutant, or LTK63, a completely nontoxic mutant, as the adjuvant. Each of the meningococcal proteins induced significant cellular responses; NhhA and NadA induced strong antibody responses, but only NadA induced bactericidal antibody when administered intranasally with mucosal adjuvants. In addition, immunoglobulin A and bactericidal antibodies were detected in the respiratory tract following intranasal delivery of NadA. Analysis of antigen-specific cytokine production by T cells from immunized mice revealed that intranasal immunization with NadA alone failed to generate detectable cellular immune responses. In contrast, LTK63, LTR72, and LTwt significantly augmented NadA-specific gamma interferon, interleukin-4 (IL-4), IL-5, and IL-10 production by spleen and lymph node cells, suggesting that both Th1 and Th2 cells were induced in vivo. The strongest cellular responses and highest bactericidal antibody titers were generated with LTR72 as the adjuvant. These findings demonstrate that the quality and magnitude of the immune responses generated by mucosal vaccines are influenced by the antigen as well as the adjuvant and suggest that nasal delivery of NadA with mucosal adjuvants has considerable potential in the development of a mucosal vaccine against serogroup B meningococci.
Figures




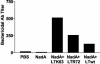
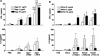
References
-
- Burton, D. R., L. Gregory, and R. Jefferis. 1986. Aspects of the molecular structure of IgG subclasses. Monogr. Allergy 19:7-35. - PubMed
-
- Cadoz, M. 1998. Potential and limitations of polysaccharide vaccines in infancy. Vaccine 16:1391-1395. - PubMed
-
- Cartwright, K., N. Noah, and H. Peltola. 2001. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European advisory board meeting Vienna, Austria, 6-8 October, 2000. Vaccine 19:4347-4356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

