Bone marrow norepinephrine mediates development of functionally different macrophages after thermal injury and sepsis
- PMID: 15213629
- PMCID: PMC1356385
- DOI: 10.1097/01.sla.0000130724.84914.d6
Bone marrow norepinephrine mediates development of functionally different macrophages after thermal injury and sepsis
Abstract
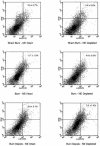
Objective: We sought to determine the influence of thermal (burn) injury with sepsis and norepinephrine on the clonogenic potential and functional cytokine response to lipopolysaccharide (LPS) stimulation in nonmyeloid committed (CD117) and myeloid committed (ER-MP12) bone marrow progenitor cells.
Summary and background data: We have previously demonstrated that norepinephrine stimulated myelopoiesis after burn injury and sepsis, but the site of this stimulation in monocyte development is unknown. In the present study the influence of norepinephrine on the developmental hierarchy of bone marrow cells after thermal injury and sepsis was determined by assessing the clonogenic potential and LPS-stimulated cytokine responses of mature macrophages derived from CD117 and ER-MP12 bone marrow progenitor cells.
Methods: Tissue and bone marrow norepinephrine content was ablated by chemical sympathectomy with 6-hydroxydopamine treatment. CD117 and ER-MP12 bone marrow cells were isolated using antibody-linked magnetic microbeads. Clonogenic potential in response to colony-stimulating factors was determined. Both progenitor cell types were differentiated to mature macrophages in vitro and tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 cytokine responses to LPS provocation were determined.
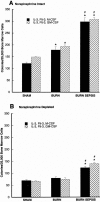
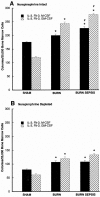
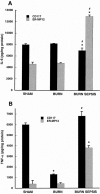
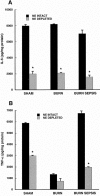
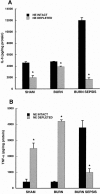
Results: The macrophage- and granulocyte-macrophage colony-stimulating factor responsive clonogenic potential was increased with burn sepsis, suggesting an expansion of both progenitor populations. Such increases were greatly reduced with prior depletion of norepinephrine. TNF-alpha and IL-6 cytokine responses to LPS were markedly influenced by the specific progenitor cells involved as well as the injury conditions and the status of norepinephrine prior to injury. In burn sepsis the depletion of norepinephrine resulted in a dramatic decrease in both IL-6 and TNF-alpha production by both progenitor-derived macrophages.
Conclusions: Depletion of norepinephrine attenuated burn and burn sepsis-induced bone marrow progenitor clonal growth in response to macrophage- and granulocyte-macrophage colony-stimulating factor. Functional phenotypes of bone marrow progenitor-derived macrophages are greatly influenced by norepinephrine and the milieu created by thermal injury and sepsis.
Figures
References
-
- Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA. 1997;278:234–240. - PubMed
-
- Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. - PubMed
-
- Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. - PubMed
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

