Patterns of amphotericin B killing kinetics against seven Candida species
- PMID: 15215097
- PMCID: PMC434204
- DOI: 10.1128/AAC.48.7.2477-2482.2004
Patterns of amphotericin B killing kinetics against seven Candida species
Abstract
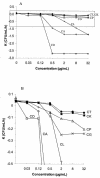
In a previous study tolerance to amphotericin B (AMB) was found among Candida parapsilosis and C. dubliniensis strains by seeding the whole volumes of wells used for MIC determinations, and minimum fungicidal concentrations (MFC) for non-C. albicans Candida strains were demonstrated to be above the levels safely achievable in serum. As an extension of that study, we performed time-kill assays with 26 blood culture isolates (6 C. albicans, 5 C. parapsilosis, 5 C. krusei, 4 C. glabrata, 3 C. lusitaniae, and 3 C. tropicalis isolates), 3 oropharyngeal C. dubliniensis isolates, 3 AMB-susceptible isolates (ATCC 90028, ATCC 22019, ATCC 6254), and 6 AMB-resistant isolates (ATCC 200955, ATCC 200956, ATCC 200950, ATCC 200951, ATCC 200952, ATCC 200953) using RPMI 1640 medium and 0.12 to 32 microg of AMB per ml and determined the numbers of CFU per milliliter at 0, 2, 4, 8, 12, 24, and 48 h. MFCs and time-kill patterns were species specific (MFCs, < or =1 microg/ml for all C. dubliniensis and C. albicans isolates except AMB-resistant strain ATCC 200955; MFCs, 2 to >16 microg/ml for the other isolates). The times required to reach the fungicidal endpoint (99.9% killing) at four times the MIC were 2 h for C. albicans and C. dubliniensis, 16 h for C. glabrata, 24 h for C. parapsilosis and C. lusitaniae, and > or =40 h for C. tropicalis and C. krusei. The killing rate increased as the AMB concentration was increased up to 2 microg/ml. The highest killing rates were achieved for C. albicans, C. dubliniensis, and C. lusitaniae, while viable C. tropicalis, C. krusei, and C. parapsilosis cells were present after 48 h (MICs, < or =2 microg/ml) when AMB was used at 2 microg/ml. Time-kill curves and MFCs can detect viable cells after 48 h when AMB is used at > or =2 microg/ml. The failure of AMB treatment could be due to its poor killing activity against some species at the concentrations reached in patients' serum.
Figures


References
-
- Burguess, S. D., R. W. Hasting, K. K. Summers, T. C. Harding, and M. G. Rinaldi. 2000. Pharmacodynamics of fluconazole, itraconazole, and amphotericin B against Candida albicans. Diagn. Microbiol. Infect. Dis. 36:13-18. - PubMed
-
- Cantón, E., and J. Pemán. 1999. Curvas de letalidad en antifúngicos. Rev. Iberoam. Micol. 16:82-85. - PubMed
-
- Cantón, E., J. Pemán, A. Viudes, G. Quindós, M. Gobernado, and A. Espinel-Ingroff. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 45:203-206. - PubMed
-
- Diekeman, D. J., M. A. Pfaller, R. N. Jones, and SENTRY Participants Group. 2002. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY Antimicrobial Surveillance Program, 1997-2000. Int. J. Antimicrob. Agents 20:412-418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

