Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers
- PMID: 15215110
- PMCID: PMC434165
- DOI: 10.1128/AAC.48.7.2570-2575.2004
Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers
Abstract
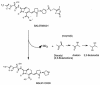
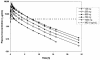
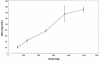
BAL5788 is the water-soluble prodrug of BAL9141, a novel broad-spectrum cephalosporin with potent bactericidal activities against methicillin-resistant Staphylococcus aureus (MRSA) and penicillin-resistant Streptococcus pneumoniae. We investigated the safety and pharmacokinetics of BAL5788 in a double-blind, single-ascending-dose study with 40 healthy male subjects. The subjects were randomized to receive placebo (n = 2 subjects per dose) or BAL5788 (n = 6 subjects per dose) as a 200-ml intravenous infusion over 30 min. The BAL5788 doses used were 125, 250, 500, 750, and 1,000 mg (BAL9141 equivalents). All doses were well tolerated, with no severe or serious adverse events (AEs). The most frequent AE was taste disturbance. No electrocardiographic abnormalities and no trends or clinically significant changes in laboratory parameters or vital signs were observed. The maximum concentration of drug in serum and the area under the concentration-time curve for BAL9141 were dose proportional over the dosing range. The elimination half-life of BAL9141 was about 3 h. The volume of distribution at steady state was equal to the volume of the adult extracellular water compartment, and the rate of renal clearance of free drug corresponded to the normal glomerular filtration rate for adults. More than 70% of the administered dose was excreted as BAL9141 in the urine, and almost no prodrug was detected. After the infusion of 750 mg, the mean plasma BAL9141 concentrations exceeded the MIC at which 100% of MRSA isolates are inhibited (4 microg/ml) for approximately 7 h, or 58% of a 12-h dosing interval. These results indicate that infusions of 750 mg twice a day should be adequate for the treatment of infections caused by MRSA.
Figures
Similar articles
-
Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers.Antimicrob Agents Chemother. 2004 Jul;48(7):2576-80. doi: 10.1128/AAC.48.7.2576-2580.2004. Antimicrob Agents Chemother. 2004. PMID: 15215111 Free PMC article. Clinical Trial.
-
Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia.Antimicrob Agents Chemother. 2004 Apr;48(4):1105-11. doi: 10.1128/AAC.48.4.1105-1111.2004. Antimicrob Agents Chemother. 2004. PMID: 15047508 Free PMC article.
-
BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis.Antimicrob Agents Chemother. 2002 Jan;46(1):171-7. doi: 10.1128/AAC.46.1.171-177.2002. Antimicrob Agents Chemother. 2002. PMID: 11751129 Free PMC article.
-
Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity.Clin Pharmacokinet. 2008;47(1):21-33. doi: 10.2165/00003088-200847010-00003. Clin Pharmacokinet. 2008. PMID: 18076216 Review.
-
Ceftaroline: a novel broad-spectrum cephalosporin with activity against meticillin-resistant Staphylococcus aureus.Drugs. 2009;69(7):809-31. doi: 10.2165/00003495-200969070-00003. Drugs. 2009. PMID: 19441869 Review.
Cited by
-
Postantibiotic effect of ceftobiprole against 12 Gram-positive organisms.Antimicrob Agents Chemother. 2006 Nov;50(11):3956-8. doi: 10.1128/AAC.00724-06. Antimicrob Agents Chemother. 2006. PMID: 17065631 Free PMC article.
-
Time-kill and synergism studies of ceftobiprole against Enterococcus faecalis, including beta-lactamase-producing and vancomycin-resistant isolates.Antimicrob Agents Chemother. 2007 Jun;51(6):2043-7. doi: 10.1128/AAC.00131-07. Epub 2007 Apr 16. Antimicrob Agents Chemother. 2007. PMID: 17438057 Free PMC article.
-
Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin.Antimicrob Agents Chemother. 2005 Oct;49(10):4210-9. doi: 10.1128/AAC.49.10.4210-4219.2005. Antimicrob Agents Chemother. 2005. PMID: 16189100 Free PMC article.
-
Antipneumococcal activity of ceftobiprole, a novel broad-spectrum cephalosporin.Antimicrob Agents Chemother. 2005 May;49(5):1932-42. doi: 10.1128/AAC.49.5.1932-1942.2005. Antimicrob Agents Chemother. 2005. PMID: 15855516 Free PMC article.
-
Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers.Antimicrob Agents Chemother. 2004 Jul;48(7):2576-80. doi: 10.1128/AAC.48.7.2576-2580.2004. Antimicrob Agents Chemother. 2004. PMID: 15215111 Free PMC article. Clinical Trial.
References
-
- Alexander, J., D. S. Bindra, J. D. Glass, M. A. Holahan, M. L. Renyer, G. S. Rork, et al. 1996. Investigation of (oxodioxolenyl)methyl carbamates as nonchiral bioreversible prodrug moieties for chiral amines. J. Med. Chem. 39:480-486. - PubMed
-
- Cars, O. 1997. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn. Microbiol. Infect. Dis. 27:29-33. - PubMed
-
- Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 2):233-237. - PubMed
-
- Dancer, S. J. 2001. The problem with cephalosporins. J. Antimicrob. Chemother. 48:463-478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

