Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers
- PMID: 15215111
- PMCID: PMC434166
- DOI: 10.1128/AAC.48.7.2576-2580.2004
Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers
Abstract
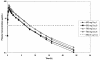
BAL5788 is the water-soluble prodrug of BAL9141, a novel broad-spectrum cephalosporin with potent bactericidal activity against methicillin-resistant Staphylococcus aureus (MRSA) and penicillin-resistant Streptococcus pneumoniae. Safety and pharmacokinetic data from a multiple-dose study with 16 healthy male volunteers are reported. Subjects were randomized to receive BAL5788 at 500 or 750 mg (as BAL9141 equivalents; n = 6 subjects per dose) or placebo (n = 2 subjects per dose). The doses were given as 200-ml infusions over 30 min once daily on days 1 and 8 and twice daily on days 2 to 7. BAL5788 was well tolerated, with no severe or serious adverse events (AEs) or dosing-related changes in laboratory parameters, electrocardiographic findings, or vital signs. Drug accumulation in plasma was negligible during the dosing period. The results of pharmacokinetic analyses agreed well with data reported from a previous single-ascending-dose study. The elimination half-life of BAL9141 was about 3 h. The volume of distribution at steady state was equal to the volume of the adult extracellular water compartment. BAL9141 was predominantly eliminated in urine, and renal clearance of the free drug corresponded to the normal glomerular filtration rate in adults. After multiple infusions of 750 mg, the mean concentrations of BAL9141 in plasma exceeded the MIC at which 100% of MRSA isolates are inhibited (4 microg/ml) for approximately 7 to 9 h, corresponding to 58 to 75% of a 12-h dosing interval.
Figures
References
-
- Alexander, J., D. S. Bindra, J. D. Glass, M. A. Holahan, M. L. Renyer, G. S. Rork, et al. 1996. Investigation of (oxodioxolenyl) methyl carbamates as nonchiral bioreversible prodrug moieties for chiral amines. J. Med. Chem. 39:480-486. - PubMed
-
- Cars, O. 1997. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn. Microbiol. Infect. Dis. 27:29-33. - PubMed
-
- Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 2):233-237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources