Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms
- PMID: 15215123
- PMCID: PMC434183
- DOI: 10.1128/AAC.48.7.2659-2664.2004
Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms
Abstract
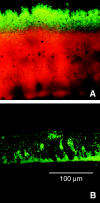
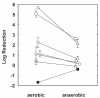
The role of oxygen limitation in protecting Pseudomonas aeruginosa strains growing in biofilms from killing by antibiotics was investigated in vitro. Bacteria in mature (48-h-old) colony biofilms were poorly killed when they were exposed to tobramycin, ciprofloxacin, carbenicillin, ceftazidime, chloramphenicol, or tetracycline for 12 h. It was shown with oxygen microelectrodes that these biofilms contain large anoxic regions. Oxygen penetrated about 50 microm into the biofilms, which averaged 210 microm thick. The region of active protein synthesis was visualized by using an inducible green fluorescent protein. This zone was also limited to a narrow band, approximately 30 microm wide, adjacent to the air interface of the biofilm. The bacteria in mature biofilms exhibited a specific growth rate of only 0.02 h(-1). These results show that 48-h-old colony biofilms are physiologically heterogeneous and that most of the cells in the biofilm occupy an oxygen-limited, stationary-phase state. In contrast, bacteria in 4-h-old colony biofilms were still growing, active, and susceptible to antibiotics when they were challenged in air. When 4-h-old colony biofilms were challenged under anaerobic conditions, the level of killing by antibiotics was reduced compared to that for the controls grown aerobically. Oxygen limitation could explain 70% or more of the protection afforded to 48-h-old colony biofilms for all antibiotics tested. Nitrate amendment stimulated the growth of untreated control P. aeruginosa isolates grown under anaerobic conditions but decreased the susceptibilities of the organisms to antibiotics. Local oxygen limitation and the presence of nitrate may contribute to the reduced susceptibilities of P. aeruginosa biofilms causing infections in vivo.
Figures
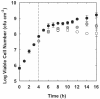

 , chloramphenicol; ◊, tetracycline).
, chloramphenicol; ◊, tetracycline).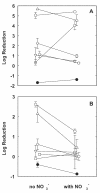
 , chloramphenicol; ◊, tetracycline.
, chloramphenicol; ◊, tetracycline.References
-
- Bagge, N., O. Ciofu, L. T. Skovgaard, and N. Hoiby. 2000. Rapid development in vitro and in vivo of resistance to ceftazidime in biofilm-growing Pseudomonas aeruginosa due to chromosomal beta-lactamase. APMIS 108:589-600. - PubMed
-
- Brown, M. R. W., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-783. - PubMed
-
- Bryant, R. E., and J. A. Mazza. 1989. Effect of the abscess environment on the antimicrobial activity of ciprofloxacin. Am. J. Med. 87(Suppl. 5A):23S-27S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

