Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts
- PMID: 15215160
- PMCID: PMC1618540
- DOI: 10.1016/S0002-9440(10)63273-7
Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts
Abstract
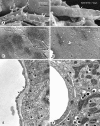
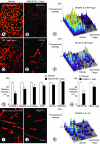
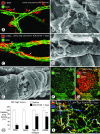
Angiogenesis inhibitors are receiving increased attention as cancer therapeutics, but little is known of the cellular effects of these inhibitors on tumor vessels. We sought to determine whether two agents, AG013736 and VEGF-Trap, that inhibit vascular endothelial growth factor (VEGF) signaling, merely stop angiogenesis or cause regression of existing tumor vessels. Here, we report that treatment with these inhibitors caused robust and early changes in endothelial cells, pericytes, and basement membrane of vessels in spontaneous islet-cell tumors of RIP-Tag2 transgenic mice and in subcutaneously implanted Lewis lung carcinomas. Strikingly, within 24 hours, endothelial fenestrations in RIP-Tag2 tumors disappeared, vascular sprouting was suppressed, and patency and blood flow ceased in some vessels. By 7 days, vascular density decreased more than 70%, and VEGFR-2 and VEGFR-3 expression was reduced in surviving endothelial cells. Vessels in Lewis lung tumors, which lacked endothelial fenestrations, showed less regression. In both tumors, pericytes did not degenerate to the same extent as endothelial cells, and those on surviving tumor vessels acquired a more normal phenotype. Vascular basement membrane persisted after endothelial cells degenerated, providing a ghost-like record of pretreatment vessel number and location and a potential scaffold for vessel regrowth. The potent anti-vascular action observed is evidence that VEGF signaling inhibitors do more than stop angiogenesis. Early loss of endothelial fenestrations in RIP-Tag2 tumors is a clue that vessel phenotype may be predictive of exceptional sensitivity to these inhibitors.
Figures



References
-
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94:883–893. - PubMed
-
- Herbst RS, Mullani NA, Davis DW, Hess KR, McConkey DJ, Charnsangavej C, O’Reilly MS, Kim HW, Baker C, Roach J, Ellis LM, Rashid A, Pluda J, Bucana C, Madden TL, Tran HT, Abbruzzese JL. Development of biologic markers of response and assessment of antiangiogenic activity in a clinical trial of human recombinant endostatin. J Clin Oncol. 2002;20:3804–3814. - PubMed
-
- Ellis LM. Antiangiogenic therapy: more promise and, yet again, more questions. J Clin Oncol. 2003;21:3897–3899. - PubMed
-
- McCarthy M. Antiangiogenesis drug promising for metastatic colorectal cancer. Lancet. 2003;361:1959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

