All-trans-retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture
- PMID: 15215172
- PMCID: PMC1618544
- DOI: 10.1016/S0002-9440(10)63285-3
All-trans-retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture
Abstract
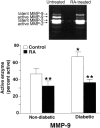
Diabetes increases susceptibility to chronic skin ulceration. The etiology of chronic wound formation in diabetic individuals is multifactoral but may be accelerated by changes in the structure and function of the skin secondary to impaired fibroblast proliferation, decreased collagen synthesis, and increased matrix metalloproteinase (MMP) expression. This study explored the effects of all-trans-retinoic acid (RA) on cellular and biochemical features of diabetic human skin in organ culture. Two-mm skin biopsies from hip or ankle were obtained from diabetic subjects and incubated for 9 days in the absence or presence of 2 micro mol/L RA. Hip skin from non-diabetic individuals served as control. Following organ culture incubation, untreated and RA-treated tissue was examined histologically after staining with hematoxylin and eosin. In parallel, organ culture-conditioned medium collected on days 5 and 7 was assayed for levels of active and total MMP-1 (interstitial collagenase) and MMP-9 (gelatinase B). The same organ culture fluids were assayed for the presence of soluble collagen. In comparison with skin from non-diabetic individuals, diabetic skin demonstrated no major differences in overall epidermal thickness or collagen production (both were increased in RA-treated tissue as compared to non-RA-treated tissue). In contrast, levels of MMP-9 (active forms) were elevated in organ culture fluid from diabetic skin as compared to non-diabetic control skin. In the presence of RA, active forms of both MMP-1 and MMP-9 were reduced. Together, these data suggest that RA has the capacity to improve structure and function of diabetic skin, and that a major effect is on reduction of collagen-degrading MMPs.
Figures




Similar articles
-
All-trans retinoic acid improves structure and function of diabetic rat skin in organ culture.Diabetes. 2002 Dec;51(12):3510-6. doi: 10.2337/diabetes.51.12.3510. Diabetes. 2002. PMID: 12453908
-
Matrix metalloproteinases in mild and severe temporomandibular joint internal derangement synovial fluid.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001 May;91(5):517-25. doi: 10.1067/moe.2001.115136. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001. PMID: 11346728
-
Expression of serine proteinases and metalloproteinases in organ-cultured human skin. Altered levels in the presence of retinoic acid and possible relationship to retinoid-induced loss of epidermal cohesion.Am J Pathol. 1994 Sep;145(3):561-73. Am J Pathol. 1994. PMID: 8080040 Free PMC article.
-
The role of matrix metalloproteinases in diabetes mellitus.Curr Top Med Chem. 2012;12(10):1159-65. doi: 10.2174/1568026611208011159. Curr Top Med Chem. 2012. PMID: 22519446 Review.
-
Epithelial cell invasion of the stroma in human skin organ culture.Front Biosci. 2004 Sep 1;9:2989-95. doi: 10.2741/1453. Front Biosci. 2004. PMID: 15353331 Review.
Cited by
-
Wound Induced Hair Neogenesis - A Novel Paradigm for Studying Regeneration and Aging.Front Cell Dev Biol. 2020 Oct 15;8:582346. doi: 10.3389/fcell.2020.582346. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33178696 Free PMC article. Review.
-
MDI 301, a nonirritating retinoid, improves abrasion wound healing in damaged/atrophic skin.Wound Repair Regen. 2008 Jan-Feb;16(1):117-24. doi: 10.1111/j.1524-475X.2007.00338.x. Wound Repair Regen. 2008. PMID: 18211583 Free PMC article.
-
The Göttingen minipig for assessment of retinoid efficacy in the skin: comparison of results from topically treated animals with results from organ-cultured skin.In Vitro Cell Dev Biol Anim. 2009 Oct;45(9):551-7. doi: 10.1007/s11626-009-9221-6. Epub 2009 Jun 18. In Vitro Cell Dev Biol Anim. 2009. PMID: 19536603 Free PMC article.
-
Effects of a synthetic retinoid on skin structure, matrix metalloproteinases, and procollagen in healthy and high-risk subjects with diabetes.J Diabetes Complications. 2011 Nov-Dec;25(6):398-404. doi: 10.1016/j.jdiacomp.2011.10.002. Epub 2011 Nov 4. J Diabetes Complications. 2011. PMID: 22055260 Free PMC article.
-
Chronic low-dose isotretinoin treatment limits renal damage in subtotally nephrectomized rats.J Mol Med (Berl). 2009 Jan;87(1):53-64. doi: 10.1007/s00109-008-0404-5. Epub 2008 Sep 16. J Mol Med (Berl). 2009. PMID: 18795249
References
-
- Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. Bethesda, MD: National Diabetes Data Group, National Institutes of Health,; Diabetes in America, (ed. 2) 1995:pp 409–428.
-
- Reiber GE. The epidemiology of diabetic foot problems. Diabetic Med. 1998;13:S6–S11. - PubMed
-
- Margolis D, Hoffstad O. Diabetic neuropathic foot ulcers. Diabetes Care. 2002;25:10. - PubMed
-
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment: a meta-analysis. Diabetes Care. 1999;22:692–695. - PubMed
-
- Eagelton WP. Influence of old age, diabetes, arterial sclerosis and gout on the healing of wounds. Am Med. 1902;4:898–904.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

