Adenosine down-regulates the surface expression of dipeptidyl peptidase IV on HT-29 human colorectal carcinoma cells: implications for cancer cell behavior
- PMID: 15215186
- PMCID: PMC1618535
- DOI: 10.1016/S0002-9440(10)63299-3
Adenosine down-regulates the surface expression of dipeptidyl peptidase IV on HT-29 human colorectal carcinoma cells: implications for cancer cell behavior
Abstract
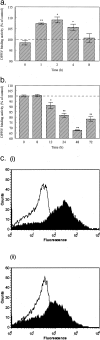
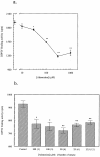
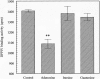
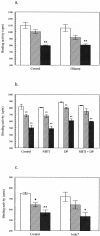
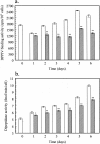
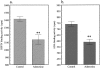
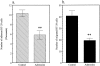
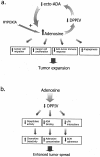
Dipeptidyl peptidase IV (DPPIV) is a multifunctional cell-surface protein that, as well as having dipeptidase activity, is the major binding protein for adenosine deaminase (ADA) and also binds extracellular matrix proteins such as fibronectin and collagen. It typically reduces the activity of chemokines and other peptide mediators as a result of its enzymatic activity. DPPIV is aberrantly expressed in many cancers, and decreased expression has been linked to increases in invasion and metastasis. We asked whether adenosine, a purine nucleoside that is present at increased levels in the hypoxic tumor microenvironment, might affect the expression of DPPIV at the cell surface. Treatment with a single dose of adenosine produced an initial transient (1 to 4 hours) modest (approximately 10%) increase in DPPIV, followed by a more profound (approximately 40%) depression of DPPIV protein expression at the surface of HT-29 human colon carcinoma cells, with a maximal decline being reached after 48 hours, and persisting for at least a week with daily exposure to adenosine. This down-regulation ofDPPIV occurred at adenosine concentrations comparable to those present within the extracellular fluid of colorectal tumors growing in vivo, and was not elicited by inosine or guanosine. Neither cellular uptake of adenosine nor its phosphorylation was necessary for the down-regulation of DPPIV. The decrease in DPPIV protein at the cell surface was paralleled by decreases in DPPIV enzyme activity, binding of ADA, and the ability of the cells to bind to and migrate on cellular fibronectin. Adenosine, at concentrations that exist within solid tumors, therefore acts at the surface of colorectal carcinoma cells to decrease levels and activities of DPPIV. This down-regulation of DPPIV may increase the sensitivity of cancer cells to the tumor-promoting effects of adenosine and their response to chemokines and the extracellular matrix, facilitating their expansion and metastasis.
Figures


Similar articles
-
Adenosine downregulates DPPIV on HT-29 colon cancer cells by stimulating protein tyrosine phosphatase(s) and reducing ERK1/2 activity via a novel pathway.Am J Physiol Cell Physiol. 2006 Sep;291(3):C433-44. doi: 10.1152/ajpcell.00238.2005. Epub 2006 Apr 12. Am J Physiol Cell Physiol. 2006. PMID: 16611738
-
N-linked glycosylation of dipeptidyl peptidase IV (CD26): effects on enzyme activity, homodimer formation, and adenosine deaminase binding.Protein Sci. 2004 Jan;13(1):145-54. doi: 10.1110/ps.03352504. Protein Sci. 2004. PMID: 14691230 Free PMC article.
-
Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain.Eur J Biochem. 1999 Dec;266(3):798-810. doi: 10.1046/j.1432-1327.1999.00902.x. Eur J Biochem. 1999. PMID: 10583373
-
Molecular mechanism and structural basis of interactions of dipeptidyl peptidase IV with adenosine deaminase and human immunodeficiency virus type-1 transcription transactivator.Eur J Cell Biol. 2012 Apr;91(4):265-73. doi: 10.1016/j.ejcb.2011.06.001. Epub 2011 Sep 28. Eur J Cell Biol. 2012. PMID: 21856036 Review.
-
The role of CD26/dipeptidyl peptidase IV in cancer.Front Biosci. 2008 Jan 1;13:1634-45. doi: 10.2741/2787. Front Biosci. 2008. PMID: 17981655 Review.
Cited by
-
Cancer biomarker discovery: the entropic hallmark.PLoS One. 2010 Aug 18;5(8):e12262. doi: 10.1371/journal.pone.0012262. PLoS One. 2010. PMID: 20805891 Free PMC article.
-
DPPIV/CD26: a tumor suppressor or a marker of malignancy?Tumour Biol. 2016 Jun;37(6):7059-73. doi: 10.1007/s13277-016-5005-2. Epub 2016 Mar 4. Tumour Biol. 2016. PMID: 26943912 Review.
-
Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers?Front Pharmacol. 2021 May 18;12:681477. doi: 10.3389/fphar.2021.681477. eCollection 2021. Front Pharmacol. 2021. PMID: 34084146 Free PMC article. Review.
-
The Adenosine System at the Crossroads of Intestinal Inflammation and Neoplasia.Int J Mol Sci. 2020 Jul 18;21(14):5089. doi: 10.3390/ijms21145089. Int J Mol Sci. 2020. PMID: 32708507 Free PMC article. Review.
-
Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go?Purinergic Signal. 2013 Jun;9(2):145-65. doi: 10.1007/s11302-012-9349-9. Epub 2012 Dec 28. Purinergic Signal. 2013. PMID: 23271562 Free PMC article. Review.
References
-
- Dinjen WNM, Ten Kate J, van der Linden EPM, Wijnen JT, Khan PM, Bosman FT. Distribution of adenosine deaminase complexing protein (ADCP) in human tissues. J Histochem Cytochem. 1989;37:1869–1875. - PubMed
-
- Morimoto C, Schlossman S. The structure and function of CD26 in the T-cell immune response. Immunol Rev. 1998;61:55–70. - PubMed
-
- De Meester I, Korom S, Van Damme J, Scharpe S. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–375. - PubMed
-
- Drucker DJ. The glucagon-like peptides. Endocrinology. 2001;142:521–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

