Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules
- PMID: 15215300
- PMCID: PMC6729225
- DOI: 10.1523/JNEUROSCI.1382-04.2004
Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules
Abstract
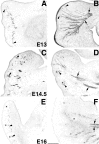
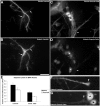
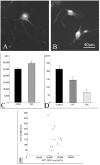
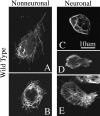
Katanin is a heterodimeric enzyme that severs microtubules from the centrosome so that they can move into the axon. Katanin is broadly distributed in the neuron, and therefore presumably also severs microtubules elsewhere. Such severing would generate multiple short microtubules from longer microtubules, resulting in more microtubule ends available for assembly and interaction with other structures. In addition, shorter microtubules are thought to move more rapidly and undergo organizational changes more readily than longer microtubules. In dividing cells, the levels of P60-katanin (the subunit with severing properties) increase as the cell transitions from interphase to mitosis. This suggests that katanin is regulated in part by its absolute levels, given that katanin activity is high during mitosis. In the rodent brain, neurons vary significantly in katanin levels, depending on their developmental stage. Levels are high during rapid phases of axonal growth but diminish as axons reach their targets. Similarly, in neuronal cultures, katanin levels are high when axons are allowed to grow avidly but drop when the axons are presented with target cells that cause them to stop growing. Expression of a dominant-negative P60-katanin construct in cultured neurons inhibits microtubule severing and is deleterious to axonal growth. Overexpression of wild-type P60-katanin results in excess microtubule severing and is also deleterious to axonal growth, but this only occurs in some neurons. Other neurons are relatively unaffected by overexpression. Collectively, these observations indicate that axonal growth is sensitive to the levels of P60-katanin, but that other factors contribute to modulating this sensitivity.
Figures





Similar articles
-
An essential role for katanin in severing microtubules in the neuron.J Cell Biol. 1999 Apr 19;145(2):305-15. doi: 10.1083/jcb.145.2.305. J Cell Biol. 1999. PMID: 10209026 Free PMC article.
-
The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches.Mol Biol Cell. 2008 Apr;19(4):1485-98. doi: 10.1091/mbc.e07-09-0878. Epub 2008 Jan 30. Mol Biol Cell. 2008. PMID: 18234839 Free PMC article.
-
Regulation of microtubule severing by katanin subunits during neuronal development.J Neurosci. 2005 Jun 8;25(23):5573-83. doi: 10.1523/JNEUROSCI.0834-05.2005. J Neurosci. 2005. PMID: 15944385 Free PMC article.
-
Cellular Samurai: katanin and the severing of microtubules.J Cell Sci. 2000 Aug;113 ( Pt 16):2821-7. doi: 10.1242/jcs.113.16.2821. J Cell Sci. 2000. PMID: 10910766 Review.
-
[Role of microtubule severing proteins in cytoskeleton reorganization].Postepy Biochem. 2016;62(1):52-59. Postepy Biochem. 2016. PMID: 28132445 Review. Polish.
Cited by
-
Glial response to hypoxia in mutants of NPAS1/3 homolog Trachealess through Wg signaling to modulate synaptic bouton organization.PLoS Genet. 2019 Aug 5;15(8):e1007980. doi: 10.1371/journal.pgen.1007980. eCollection 2019 Aug. PLoS Genet. 2019. PMID: 31381576 Free PMC article.
-
Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases.Int J Mol Sci. 2020 Oct 5;21(19):7354. doi: 10.3390/ijms21197354. Int J Mol Sci. 2020. PMID: 33027950 Free PMC article. Review.
-
Tau protects microtubules in the axon from severing by katanin.J Neurosci. 2006 Mar 22;26(12):3120-9. doi: 10.1523/JNEUROSCI.5392-05.2006. J Neurosci. 2006. PMID: 16554463 Free PMC article.
-
Evaluation of loss of function as an explanation for SPG4-based hereditary spastic paraplegia.Hum Mol Genet. 2010 Jul 15;19(14):2767-79. doi: 10.1093/hmg/ddq177. Epub 2010 Apr 29. Hum Mol Genet. 2010. PMID: 20430936 Free PMC article.
-
Gene dosage-dependent rescue of HSP neurite defects in SPG4 patients' neurons.Hum Mol Genet. 2014 May 15;23(10):2527-41. doi: 10.1093/hmg/ddt644. Epub 2013 Dec 30. Hum Mol Genet. 2014. PMID: 24381312 Free PMC article.
References
-
- Ahmad FJ, Joshi HC, Centonze VE, Baas PW (1994) Inhibition of microtubule nucleation at the neuronal centrosome compromises axon growth. Neuron 12: 271-280. - PubMed
-
- Ahmad FJ, Hughey J, Wittmann T, Hyman A, Greaser M, Baas PW (2000) Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat Cell Biol 2: 276-280. - PubMed
-
- Avila J, Dominguez J, Diaz-Nido J (1994) Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol 38: 13-25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
