The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering
- PMID: 15215304
- PMCID: PMC6729214
- DOI: 10.1523/JNEUROSCI.1184-04.2004
The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering
Abstract
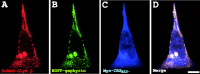
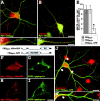
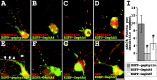
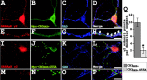
Glycine receptors (GlyRs) and specific subtypes of GABA(A) receptors are clustered at synapses by the multidomain protein gephyrin, which in turn is translocated to the cell membrane by the GDP-GTP exchange factor collybistin. We report the characterization of several new variants of collybistin, which are created by alternative splicing of exons encoding an N-terminal src homology 3 (SH3) domain and three alternate C termini (CB1, CB2, and CB3). The presence of the SH3 domain negatively regulates the ability of collybistin to translocate gephyrin to submembrane microaggregates in transfected mammalian cells. Because the majority of native collybistin isoforms appear to harbor the SH3 domain, this suggests that collybistin activity may be regulated by protein-protein interactions at the SH3 domain. We localized the binding sites for collybistin and the GlyR beta subunit to the C-terminal MoeA homology domain of gephyrin and show that multimerization of this domain is required for collybistin-gephyrin and GlyR-gephyrin interactions. We also demonstrate that gephyrin clustering in recombinant systems and cultured neurons requires both collybistin-gephyrin interactions and an intact collybistin pleckstrin homology domain. The vital importance of collybistin for inhibitory synaptogenesis is underlined by the discovery of a mutation (G55A) in exon 2 of the human collybistin gene (ARHGEF9) in a patient with clinical symptoms of both hyperekplexia and epilepsy. The clinical manifestation of this collybistin missense mutation may result, at least in part, from mislocalization of gephyrin and a major GABA(A) receptor subtype.
Figures



References
-
- Andrew M, Owen MJ (1997) Hyperekplexia: abnormal startle response due to glycine receptor mutations. Br J Psychiatry 170: 106-108. - PubMed
-
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA (1998) A novel regulator of p21-activated kinases. J Biol Chem 273: 23633-23636. - PubMed
-
- Erickson JW, Cerione RA (2001) Multiple roles for Cdc42 in cell regulation. Curr Opin Cell Biol 13: 153-157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
