Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network
- PMID: 15215310
- PMCID: PMC515336
- DOI: 10.1091/mbc.e03-12-0876
Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network
Abstract
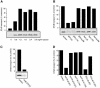
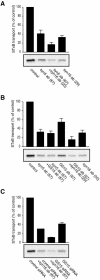
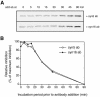
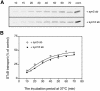
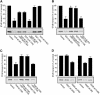
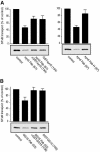
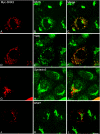
An in vitro transport assay, established with a modified Shiga toxin B subunit (STxB) as a marker, has proved to be useful for the study of transport from the early/recycling endosome (EE/RE) to the trans-Golgi network (TGN). Here, we modified this assay to test antibodies to all known soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) that have been shown to localize in the Golgi and found that syntaxin 5, GS28, Ykt6, and GS15 antibodies specifically inhibited STxB transport. Because syntaxin 5, GS28, Ykt6, and GS15 exist as a unique SNARE complex, our observation indicates that these four SNAREs function as a complex in EE/RE-TGN transport. The importance of GS15 in EE/RE-TGN transport was further demonstrated by a block in recombinant STxB transport in HeLa cells when GS15 expression was knocked down by its small interfering iRNA. Morphological analyses showed that some GS15 and Ykt6 were redistributed from the Golgi to the endosomes when the recycling endosome was perturbed by SNX3-overexpression, suggesting that GS15 and Ykt6 might cycle between the endosomes and the Golgi apparatus. Further studies indicated that syntaxin 5 and syntaxin 16 exerted their role in EE/RE-TGN transport in an additive manner. The kinetics of inhibition exhibited by syntaxin 16 and syntaxin 5 antibodies is similar.
Figures

Similar articles
-
GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus.Mol Biol Cell. 2002 Oct;13(10):3493-507. doi: 10.1091/mbc.e02-01-0004. Mol Biol Cell. 2002. PMID: 12388752 Free PMC article.
-
Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform.J Cell Biol. 2002 Feb 18;156(4):653-64. doi: 10.1083/jcb.200110081. Epub 2002 Feb 11. J Cell Biol. 2002. PMID: 11839770 Free PMC article.
-
Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport.J Biol Chem. 2001 Jul 20;276(29):27480-7. doi: 10.1074/jbc.M102786200. Epub 2001 Apr 25. J Biol Chem. 2001. PMID: 11323436
-
Syntaxin 16's Newly Deciphered Roles in Autophagy.Cells. 2019 Dec 17;8(12):1655. doi: 10.3390/cells8121655. Cells. 2019. PMID: 31861136 Free PMC article. Review.
-
Transport according to GARP: receiving retrograde cargo at the trans-Golgi network.Trends Cell Biol. 2011 Mar;21(3):159-67. doi: 10.1016/j.tcb.2010.11.003. Epub 2010 Dec 21. Trends Cell Biol. 2011. PMID: 21183348 Free PMC article. Review.
Cited by
-
Monoubiquitination of Syntaxin 5 Regulates Golgi Membrane Dynamics during the Cell Cycle.Dev Cell. 2016 Jul 11;38(1):73-85. doi: 10.1016/j.devcel.2016.06.001. Dev Cell. 2016. PMID: 27404360 Free PMC article.
-
The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network.Mol Biol Cell. 2007 Dec;18(12):4979-91. doi: 10.1091/mbc.e07-06-0622. Epub 2007 Oct 3. Mol Biol Cell. 2007. PMID: 17914056 Free PMC article.
-
Lamellipodium extension and membrane ruffling require different SNARE-mediated trafficking pathways.BMC Cell Biol. 2010 Aug 10;11:62. doi: 10.1186/1471-2121-11-62. BMC Cell Biol. 2010. PMID: 20698987 Free PMC article.
-
Another longin SNARE for autophagosome-lysosome fusion-how does Ykt6 work?Autophagy. 2019 Feb;15(2):352-357. doi: 10.1080/15548627.2018.1532261. Epub 2018 Oct 13. Autophagy. 2019. PMID: 30290706 Free PMC article.
-
Population distribution analyses reveal a hierarchy of molecular players underlying parallel endocytic pathways.PLoS One. 2014 Jun 27;9(6):e100554. doi: 10.1371/journal.pone.0100554. eCollection 2014. PLoS One. 2014. PMID: 24971745 Free PMC article.
References
-
- Bennett, M., Garcia-Arraras, J., Elferink, L., Peterson, K., Fleming, A., Hazuka, C., and Scheller, R. (1993). The syntaxin family of vesicular transport receptors. Cell 74, 863-873. - PubMed
-
- Bock, J.B., Lin, R.C., and Scheller, R.H. (1996). A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 271, 17961-17965. - PubMed
-
- Dascher, C., Matteson, J., and Balch, W. (1994). Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J. Biol. Chem. 269, 29363-29366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

