Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42
- PMID: 15215311
- PMCID: PMC491849
- DOI: 10.1091/mbc.e04-01-0019
Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42
Abstract
Axon extension during development is guided by many factors, but the signaling mechanisms responsible for its regulation remain largely unknown. We have now investigated the role of the transmembrane protein CD47 in this process in N1E-115 neuroblastoma cells. Forced expression of CD47 induced the formation of neurites and filopodia. Furthermore, an Fc fusion protein containing the extracellular region of the CD47 ligand SHPS-1 induced filopodium formation, and this effect was enhanced by CD47 overexpression. SHPS-1-Fc also promoted neurite and filopodium formation triggered by serum deprivation. Inhibition of Rac or Cdc42 preferentially blocked CD47-induced formation of neurites and filopodia, respectively. Overexpression of CD47 resulted in the activation of both Rac and Cdc42. The extracellular region of CD47 was sufficient for the induction of neurite formation by forced expression, but the entire structure of CD47 was required for enhancement of filopodium formation by SHPS-1-Fc. Neurite formation induced by CD47 was also inhibited by a mAb to the integrin beta3 subunit. These results indicate that the interaction of SHPS-1 with CD47 promotes neurite and filopodium formation through the activation of Rac and Cdc42, and that integrins containing the beta3 subunit participate in the effect of CD47 on neurite formation.
Figures
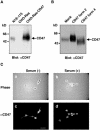

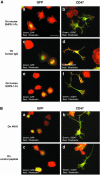
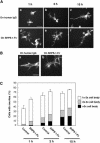


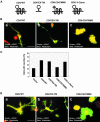
References
-
- Brown, E.J., and Frazier, W.A. (2001). Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11, 130-135. - PubMed
-
- Chung, J., Gao, A.G., and Frazier, W.A. (1997). Thrombospondin acts via integrin-associated protein to activate the platelet integrin αIIbβ3. J. Biol. Chem. 272, 14740-14746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

