A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division
- PMID: 15215313
- PMCID: PMC515334
- DOI: 10.1091/mbc.e04-01-0053
A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division
Abstract
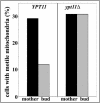
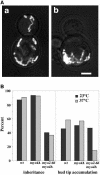
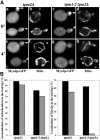
Two actin-dependent force generators contribute to mitochondrial inheritance: Arp2/3 complex and the myosin V Myo2p (together with its Rab-like binding partner Ypt11p). We found that deletion of YPT11, reduction of the length of the Myo2p lever arm (myo2-Delta6IQ), or deletion of MYO4 (the other yeast myosin V), had no effect on mitochondrial morphology, colocalization of mitochondria with actin cables, or the velocity of bud-directed mitochondrial movement. In contrast, retention of mitochondria in the bud was compromised in YPT11 and MYO2 mutants. Retention of mitochondria in the bud tip of wild-type cells results in a 60% decrease in mitochondrial movement in buds compared with mother cells. In ypt11Delta mutants, however, the level of mitochondrial motility in buds was similar to that observed in mother cells. Moreover, the myo2-66 mutant, which carries a temperature-sensitive mutation in the Myo2p motor domain, exhibited a 55% decrease in accumulation of mitochondria in the bud tip, and an increase in accumulation of mitochondria at the retention site in the mother cell after shift to restrictive temperatures. Finally, destabilization of actin cables and the resulting delocalization of Myo2p from the bud tip had no significant effect on the accumulation of mitochondria in the bud tip.
Figures



References
-
- Beach, D.L., Thibodeaux, J., Maddox, P., Yeh, E., and Bloom, K. (2000). The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol. 10, 1497-1506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

