The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium
- PMID: 15215314
- PMCID: PMC515348
- DOI: 10.1091/mbc.e04-01-0058
The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium
Abstract
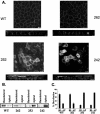
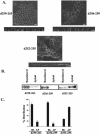
CD147, a type I integral membrane protein of the immunoglobulin superfamily, exhibits reversed polarity in retinal pigment epithelium (RPE). CD147 is apical in RPE in contrast to its basolateral localization in extraocular epithelia. This elicited our interest in understanding the basolateral sorting signals of CD147 in prototypic Madin-Darby canine kidney (MDCK) cells. The cytoplasmic domain of CD147 has basolateral sorting information but is devoid of well-characterized basolateral signals, such as tyrosine and di-leucine motifs. Hence, we carried out systematic site-directed mutagenesis to delineate basolateral targeting information in CD147. Our detailed analysis identified a single leucine (252) as the basolateral targeting motif in the cytoplasmic tail of CD147. Four amino acids (243-246) N-terminal to leucine 252 are also critical basolateral determinants of CD147, because deletion of these amino acids leads to mistargeting of CD147 to the apical membranes. We ruled out the involvement of adaptor complex 1B (AP1B) in the basolateral trafficking of CD147, because LLC-PK1 cells lacking AP1B, target CD147 basolaterally. At variance with MDCK cells, the human RPE cell line ARPE-19 does not distinguish between CD147 (WT) and CD147 with leucine 252 mutated to alanine and targets both proteins apically. Thus, our study identifies an atypical basolateral motif of CD147, which comprises a single leucine and is not recognized by RPE cells. This unusual basolateral sorting signal will be useful in unraveling the specialized sorting machinery of RPE cells.
Figures











Similar articles
-
Apical polarity of N-CAM and EMMPRIN in retinal pigment epithelium resulting from suppression of basolateral signal recognition.J Cell Biol. 1998 Aug 10;142(3):697-710. doi: 10.1083/jcb.142.3.697. J Cell Biol. 1998. PMID: 9700159 Free PMC article.
-
Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells.J Biol Chem. 1998 Oct 9;273(41):26862-9. doi: 10.1074/jbc.273.41.26862. J Biol Chem. 1998. PMID: 9756932
-
A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells.J Biol Chem. 2001 Jun 22;276(25):22565-72. doi: 10.1074/jbc.M101907200. Epub 2001 Apr 18. J Biol Chem. 2001. PMID: 11312273
-
Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases.Ann N Y Acad Sci. 1998 Oct 23;857:1-12. doi: 10.1111/j.1749-6632.1998.tb10102.x. Ann N Y Acad Sci. 1998. PMID: 9917828 Review.
-
The polarity of the retinal pigment epithelium.Traffic. 2001 Dec;2(12):867-72. doi: 10.1034/j.1600-0854.2001.21202.x. Traffic. 2001. PMID: 11737824 Review.
Cited by
-
Basigin-2 is a cell surface receptor for soluble basigin ligand.J Biol Chem. 2008 Jun 27;283(26):17805-14. doi: 10.1074/jbc.M801876200. Epub 2008 Apr 22. J Biol Chem. 2008. PMID: 18434307 Free PMC article.
-
Basolateral CD147 induces hepatocyte polarity loss by E-cadherin ubiquitination and degradation in hepatocellular carcinoma progress.Hepatology. 2018 Jul;68(1):317-332. doi: 10.1002/hep.29798. Epub 2018 Mar 23. Hepatology. 2018. PMID: 29356040 Free PMC article.
-
Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia.Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16245-50. doi: 10.1073/pnas.0504419102. Epub 2005 Oct 31. Proc Natl Acad Sci U S A. 2005. PMID: 16260747 Free PMC article.
-
Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia.Traffic. 2011 Apr;12(4):483-98. doi: 10.1111/j.1600-0854.2010.01155.x. Epub 2011 Feb 1. Traffic. 2011. PMID: 21199217 Free PMC article.
-
The syntaxin 4 N terminus regulates its basolateral targeting by munc18c-dependent and -independent mechanisms.J Biol Chem. 2011 Mar 25;286(12):10834-46. doi: 10.1074/jbc.M110.186668. Epub 2011 Jan 28. J Biol Chem. 2011. PMID: 21278252 Free PMC article.
References
-
- Alizadeh, M., Wada, M., Gelfman, C.M., Handa, J.T., and Hjelmeland, L.M. (2001). Downregulation of differentiation specific gene expression by oxidative stress in ARPE-19 cells. Invest. Ophthalmol. Vis. Sci. 42, 2706-2713. - PubMed
-
- Biswas, C., and Nugent, M.A. (1987). Membrane association of collagenase stimulatory factor(s) from B-16 melanoma cells. J. Cell Biochem. 35, 247-258. - PubMed
-
- Biswas, C., Zhang, Y., DeCastro, R., Guo, H., Nakamura, T., Kataoka, H., and Nabeshima, K. (1995). The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 55, 434-439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

