Antagonistic roles of ESCRT and Vps class C/HOPS complexes in the recycling of yeast membrane proteins
- PMID: 15215319
- PMCID: PMC515352
- DOI: 10.1091/mbc.e04-05-0420
Antagonistic roles of ESCRT and Vps class C/HOPS complexes in the recycling of yeast membrane proteins
Abstract
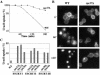
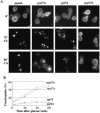
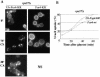
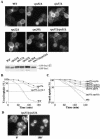
In Saccharomyces cerevisiae, deficiencies in the ESCRT machinery trigger the mistargeting of endocytic and biosynthetic ubiquitinated cargoes to the limiting membrane of the vacuole. Surprisingly, impairment of this machinery also leads to the accumulation of various receptors and transporters at the plasma membrane in both yeast and higher eukaryotes. Using the well-characterized yeast endocytic cargo uracil permease (Fur4p), we show here that the apparent stabilization of the permease at the plasma membrane in ESCRT mutants results from an efficient recycling of the protein. Whereas several proteins as well as internalized dyes are known to be recycled in yeast, little is known about the machinery and molecular mechanisms involved. The SNARE protein Snc1p is the only cargo for which the recycling pathway is well characterized. Unlike Snc1p, endocytosed Fur4p did not pass through the Golgi apparatus en route to the plasma membrane. Although ubiquitination of Fur4p is required for its internalization, deubiquitination is not required for its recycling. In an attempt to identify actors in this new recycling pathway, we found an unexpected phenotype associated with loss of function of the Vps class C complex: cells defective for this complex are impaired for recycling of Fur4p, Snc1p, and the lipophilic dye FM4-64. Genetic analyses indicated that these phenotypes were due to the functioning of the Vps class C complex in trafficking both to and from the late endosomal compartment.
Figures



Similar articles
-
Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes.Mol Biol Cell. 2000 Jan;11(1):23-38. doi: 10.1091/mbc.11.1.23. Mol Biol Cell. 2000. PMID: 10637288 Free PMC article.
-
Snc1p v-SNARE transport to the prospore membrane during yeast sporulation is dependent on endosomal retrieval pathways.Traffic. 2007 Sep;8(9):1231-45. doi: 10.1111/j.1600-0854.2007.00606.x. Epub 2007 Jul 23. Traffic. 2007. PMID: 17645731
-
Endosomal trafficking of yeast membrane proteins.Biochem Soc Trans. 2018 Dec 17;46(6):1551-1558. doi: 10.1042/BST20180258. Epub 2018 Oct 31. Biochem Soc Trans. 2018. PMID: 30381337 Free PMC article. Review.
-
Recycling of cell surface membrane proteins from yeast endosomes is regulated by ubiquitinated Ist1.J Cell Biol. 2022 Nov 7;221(11):e202109137. doi: 10.1083/jcb.202109137. Epub 2022 Sep 20. J Cell Biol. 2022. PMID: 36125415 Free PMC article.
-
Membrane trafficking in the yeast Saccharomyces cerevisiae model.Int J Mol Sci. 2015 Jan 9;16(1):1509-25. doi: 10.3390/ijms16011509. Int J Mol Sci. 2015. PMID: 25584613 Free PMC article. Review.
Cited by
-
Membrane Tethering Complexes in the Endosomal System.Front Cell Dev Biol. 2016 May 9;4:35. doi: 10.3389/fcell.2016.00035. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27243003 Free PMC article. Review.
-
Pkh1/2-dependent phosphorylation of Vps27 regulates ESCRT-I recruitment to endosomes.Mol Biol Cell. 2012 Oct;23(20):4054-64. doi: 10.1091/mbc.E12-01-0001. Epub 2012 Aug 23. Mol Biol Cell. 2012. PMID: 22918958 Free PMC article.
-
A perturbed ubiquitin landscape distinguishes between ubiquitin in trafficking and in proteolysis.Mol Cell Proteomics. 2011 May;10(5):M111.009753. doi: 10.1074/mcp.M111.009753. Epub 2011 Mar 22. Mol Cell Proteomics. 2011. PMID: 21427232 Free PMC article.
-
Ssh4, Rcr2 and Rcr1 affect plasma membrane transporter activity in Saccharomyces cerevisiae.Genetics. 2007 Apr;175(4):1681-94. doi: 10.1534/genetics.106.069716. Epub 2007 Feb 7. Genetics. 2007. PMID: 17287526 Free PMC article.
-
Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling.EMBO J. 2005 Jul 6;24(13):2265-83. doi: 10.1038/sj.emboj.7600688. Epub 2005 Jun 9. EMBO J. 2005. PMID: 15944737 Free PMC article.
References
-
- Babst, M., Odorizzi, G., Estepa, E.J., and Emr, S.D. (2000). Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1, 248-258. - PubMed
-
- Bilodeau, P.S., Urbanowski, J.L., Winistorfer, S.C., and Piper, R.C. (2002). The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4, 534-539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

