Analysis of the "thermodynamic information content" of a Homo sapiens structural database reveals hierarchical thermodynamic organization
- PMID: 15215522
- PMCID: PMC2279918
- DOI: 10.1110/ps.04706204
Analysis of the "thermodynamic information content" of a Homo sapiens structural database reveals hierarchical thermodynamic organization
Abstract
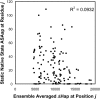
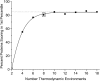
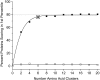
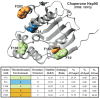
Classification of the amounts and types of lower order structural elements in proteins is a prerequisite to effective comparisons between protein folds. In an effort to provide an additional vehicle for fold comparison, we present an alternative classification scheme whereby protein folds are represented in statistical thermodynamic terms in such a way as to illuminate the energetic building blocks within protein structures. The thermodynamic relationship is examined between amino acid sequences and the conformational ensembles for a database of 159 Homo sapiens protein structures ranging from 50 to 250 amino acids. Using hierarchical clustering, it is shown through fold-recognition experiments that (1) eight thermodynamic environmental descriptors sufficiently accounts for the energetic variation within the native state ensembles of the H. sapiens structural database, (2) an amino acid library of only six residue types is sufficient to encode >90% of the thermodynamic information required for fold specificity in the entire database, and (3) structural resolution of the statistically derived environments reveals sequential cooperative segments throughout the protein, which are independent of secondary structure. As the first level of thermodynamic organization in proteins, these segments represent the thermodynamic counterpart to secondary structure.
Figures




References
-
- Anfinsen, C.B. 1973. Principles that govern the folding of protein chains. Science 181 223–230. - PubMed
-
- Babu, C.R., Hilser, V.J., and Wand, A.J. 2004. Direct access to the cooperative substructure of proteins and the protein ensemble via cold denaturation. Nat. Struct. Mol. Biol. 11 352–357. - PubMed
-
- Bai, Y. and Englander, S.W. 1996. Future directions in folding: The multi-state nature of protein structure. Proteins 24 145–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

