The human serpin proteinase inhibitor-9 self-associates at physiological temperatures
- PMID: 15215529
- PMCID: PMC2279926
- DOI: 10.1110/ps.04715304
The human serpin proteinase inhibitor-9 self-associates at physiological temperatures
Abstract
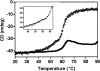
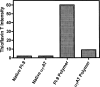
The metastable serpin architecture is perturbed by extremes of temperature, pH, or changes in primary sequence resulting in the formation of inactive, polymeric conformations. Polymerization of a number of human serpins in vivo leads to diseases such as emphysema, thrombosis, and dementia, and in these cases mutations are present within the gene encoding the aggregating protein. Here we show that aggregation of the human serpin, proteinase inhibitor-9 (PI-9), occurs under physiological conditions, and forms aggregates that are morphologically distinct from previously characterized serpin polymers. Incubation of monomeric PI-9 at 37 degrees C leads to the rapid formation of aggregated PI-9. Using a variety of spectroscopic methods we analyzed the nature of the structures formed after incubation at 37 degrees C. Electron microscopy showed that PI-9 forms ordered circular and elongated-type aggregates, which also bind the fluorescent dye Thioflavin T. Our data show that in vitro wild-type PI-9 forms aggregates at physiological temperatures. The biological implications of PI-9 aggregates at physiological temperatures are discussed.
Figures



References
-
- Bird, C.H., Sutton, V.R., Sun, J., Hirst, C.E., Novak, A., Kumar, S., Trapani, J.A., and Bird, P.I. 1998. Selective regulation of apoptosis: The cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol. Cell. Biol. 18 6387–6398. - PMC - PubMed
-
- Bladergroen, B.A., Strik, M.C., Bovenschen, N., van Berkum, O., Scheffer, G.L., Meijer, C.J., Hack, C.E., and Kummer, J.A. 2001. The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune-privileged sites. J. Immunol. 166 3218–3225. - PubMed
-
- Bottomley, S.P. and Chang, W.-S. 1997. The effect of reactive centre loop length upon serpin polymerisation. Biochem. Biophys. Res. Comm. 241 264–269. - PubMed
-
- Bottomley, S.P., Hopkins, P.C., and Whisstock, J.C. 1998. α1-antitrypsin polymerisation can occur by both loop A and C sheet mechanisms. Biochem. Biophys. Res. Comm. 251 1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

