Insulin-like growth factor (IGF)-I obliterates the pregnancy-associated protection against mammary carcinogenesis in rats: evidence that IGF-I enhances cancer progression through estrogen receptor-alpha activation via the mitogen-activated protein kinase pathway
- PMID: 15217511
- PMCID: PMC468665
- DOI: 10.1186/bcr812
Insulin-like growth factor (IGF)-I obliterates the pregnancy-associated protection against mammary carcinogenesis in rats: evidence that IGF-I enhances cancer progression through estrogen receptor-alpha activation via the mitogen-activated protein kinase pathway
Abstract
Introduction: Pregnancy protects against breast cancer development in humans and rats. Parous rats have persistently reduced circulating levels of growth hormone, which may affect the activity of the growth hormone/insulin-like growth factor (IGF)-I axis. We investigated the effects of IGF-I on parity-associated protection against mammary cancer.
Methods: Three groups of rats were evaluated in the present study: IGF-I-treated parous rats; parous rats that did not receive IGF-I treatment; and age-matched virgin animals, which also did not receive IGF-I treatment. Approximately 60 days after N-methyl-N-nitrosourea injection, IGF-I treatment was discontinued and all of the animal groups were implanted with a silastic capsule containing 17beta-estradiol and progesterone. The 17beta-estradiol plus progesterone treatment continued for 135 days, after which the animals were killed.
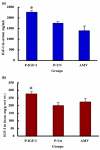
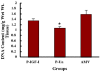
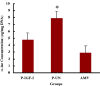
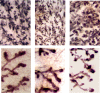
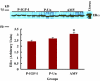
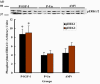
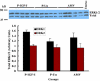
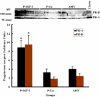
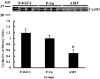
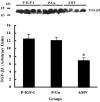
Results: IGF-I treatment of parous rats increased mammary tumor incidence to 83%, as compared with 16% in parous rats treated with 17beta-estradiol plus progesterone only. Tumor incidence and average number of tumors per animal did not differ between IGF-I-treated parous rats and age-matched virgin rats. At the time of N-methyl-N-nitrosourea exposure, DNA content was lowest but the alpha-lactalbumin concentration highest in the mammary glands of untreated parous rats in comparison with age-matched virgin and IGF-I-treated parous rats. The protein levels of estrogen receptor-alpha in the mammary gland was significantly higher in the age-matched virgin animals than in untreated parous and IGF-I-treated parous rats. Phosphorylation (activation) of the extracellular signal-regulated kinase-1/2 (ERK1/2) and expression of the progesterone receptor were both increased in IGF-I-treated parous rats, as compared with those in untreated parous and age-matched virgin rats. Expressions of cyclin D1 and transforming growth factor-beta3 in the mammary gland were lower in the age-matched virgin rats than in the untreated parous and IGF-I-treated parous rats.
Conclusion: We argue that tumor initiation (transformation and fixation of mutations) may be similar in parous and age-matched virgin animals, suggesting that the main differences in tumor formation lie in differences in tumor progression caused by the altered hormonal environment associated with parity. Furthermore, we provide evidence supporting the notion that tumor growth promotion seen in IGF-I-treated parous rats is caused by activation of estrogen receptor-alpha via the Raf/Ras/mitogen-activated protein kinase cascade.
Figures

Similar articles
-
Growth and characterization of N-methyl-N-nitrosourea-induced mammary tumors in intact and ovariectomized rats.Carcinogenesis. 2001 Dec;22(12):2039-47. doi: 10.1093/carcin/22.12.2039. Carcinogenesis. 2001. PMID: 11751437
-
Mammary tumorigenesis in growth hormone deficient spontaneous dwarf rats; effects of hormonal treatments.Breast Cancer Res Treat. 2004 Oct;87(3):277-90. doi: 10.1007/s10549-004-9504-2. Breast Cancer Res Treat. 2004. PMID: 15528971
-
Parous rats regain high susceptibility to chemically induced mammary cancer after treatment with various mammotropic hormones.Carcinogenesis. 2001 Jul;22(7):1027-33. doi: 10.1093/carcin/22.7.1027. Carcinogenesis. 2001. PMID: 11408345
-
Mechanisms of hormonal prevention of breast cancer.Ann N Y Acad Sci. 2001 Dec;952:23-35. doi: 10.1111/j.1749-6632.2001.tb02725.x. Ann N Y Acad Sci. 2001. PMID: 11795441 Review.
-
Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate.J Nutr. 2002 Mar;132(3):552S-558S. doi: 10.1093/jn/132.3.552S. J Nutr. 2002. PMID: 11880592 Review.
Cited by
-
Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells.Breast Cancer Res. 2009;11(2):R20. doi: 10.1186/bcr2245. Epub 2009 Apr 23. Breast Cancer Res. 2009. PMID: 19386118 Free PMC article.
-
The serum protein profile of early parity which induces protection against breast cancer.Oncotarget. 2016 Dec 13;7(50):82538-82553. doi: 10.18632/oncotarget.12757. Oncotarget. 2016. PMID: 27769065 Free PMC article.
-
Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models.Breast Cancer Res. 2007;9(1):R12. doi: 10.1186/bcr1645. Breast Cancer Res. 2007. PMID: 17257424 Free PMC article.
-
Functional IGF1R variant predicts breast cancer risk in women with preeclampsia in California Teachers Study.Cancer Causes Control. 2017 Oct;28(10):1027-1032. doi: 10.1007/s10552-017-0942-7. Epub 2017 Aug 18. Cancer Causes Control. 2017. PMID: 28822014 Free PMC article.
-
Interactions between IGF-I, estrogen receptor-α (ERα), and ERβ in regulating growth/apoptosis of MCF-7 human breast cancer cells.J Endocrinol. 2011 Jan;208(1):1-9. doi: 10.1677/JOE-10-0235. Epub 2010 Oct 25. J Endocrinol. 2011. PMID: 20974640 Free PMC article.
References
-
- Thordarson G, Jin E, Guzman RC, Swanson SM, Nandi S, Talamantes F. Refractoriness to mammary tumorigenesis in parous rats: is it caused by persistent changes in the hormonal environment or permanent biochemical alterations in the mammary epithelia? Carcinogenesis. 1995;16:2847–2853. - PubMed
-
- Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57:112–137. - PubMed
-
- Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2:5–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

