Mechanisms of deep brain stimulation: an intracellular study in rat thalamus
- PMID: 15218068
- PMCID: PMC1665080
- DOI: 10.1113/jphysiol.2004.064998
Mechanisms of deep brain stimulation: an intracellular study in rat thalamus
Abstract
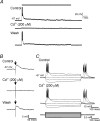
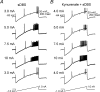
High-frequency deep brain stimulation (DBS) in the thalamus alleviates most kinds of tremor, yet its mechanism of action is unknown. Studies in subthalamic nucleus and other brain sites have emphasized non-synaptic factors. To explore the mechanism underlying thalamic DBS, we simulated DBS in vitro by applying high-frequency (125 Hz) electrical stimulation directly into the sensorimotor thalamus of adult rat brain slices. Intracellular recordings revealed two distinct types of membrane responses, both of which were initiated with a depolarization and rapid spike firing. However, type 1 responses repolarized quickly and returned to quiescent baseline during simulated DBS whereas type 2 responses maintained the level of membrane depolarization, with or without spike firing. Individual thalamic neurones exhibited either type 1 or type 2 response but not both. In all neurones tested, simulated DBS-evoked membrane depolarization was reversibly eliminated by tetrodotoxin, glutamate receptor antagonists, and the Ca(2+) channel antagonist Cd(2+). Simulated DBS also increased the excitability of thalamic cells in the presence of glutamate receptor blockade, although this non-synaptic effect induced no spontaneous firing such as that found in subthalamic nucleus neurones. Our data suggest that high-frequency stimulation when applied in the ventral thalamus can rapidly disrupt local synaptic function and neuronal firing thereby leading to a 'functional deafferentation' and/or 'functional inactivation'. These mechanisms, driven primarily by synaptic activation, help to explain the paradox that lesions, muscimol and DBS in thalamus all effectively stop tremor.
Figures







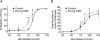
References
-
- Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003a;89:1150–1160. - PubMed
-
- Benabid AL, Pollak P, Gao DM, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorder. J Neurosurg. 1996;84:203–214. - PubMed
-
- Bergman H, Deuschl G. Pathophysiology of Parkinson's disease: from clinical neurology to basic neuroscience and back. Mov Disord. 2002;17(Suppl. 3):S28–S40. - PubMed
-
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–1356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

