Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus
- PMID: 15218072
- PMCID: PMC1665079
- DOI: 10.1113/jphysiol.2004.063016
Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus
Abstract
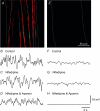
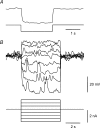
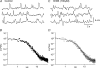
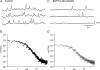
Intracellular recordings were made from isolated bundles of the circular muscle layer of mouse and guinea-pig gastric fundus. These preparations displayed an ongoing discharge of membrane noise (unitary potentials), similar to that recorded from similar preparations made from the circular layer of the antrum. Bundles of muscle from the fundus of W/W(V) mice, which lack intramuscular interstitial cells of Cajal (ICC(IM)) lacked the discharge of membrane noise observed in wild-type tissues. When the membrane potential was changed by passing depolarizing or hyperpolarizing current pulses, the discharge of membrane noise was little changed. The membrane noise was unaffected by adding chloride channel blockers; however, agents which buffered the internal concentration of calcium ions reduced the discharge of membrane noise. Treatment of tissues with CCCP, which interferes with the uptake of calcium ions by mitochondria, also reduced the membrane noise and caused membrane hyperpolarization. Similar observations were made on bundles of tissue isolated from the circular layer of the guinea pig antrum. Together the observations indicate that membrane noise is generated by a pathway located in ICC(IM). The properties of this pathway appear to vary dramatically within a given organ. The lack of voltage sensitivity of the discharge of membrane noise in the fundus provides a possible explanation for the lack of rhythmic electrical activity in this region of the stomach.
Figures




References
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

