A general approach for chemical labeling and rapid, spatially controlled protein inactivation
- PMID: 15218100
- PMCID: PMC454201
- DOI: 10.1073/pnas.0401609101
A general approach for chemical labeling and rapid, spatially controlled protein inactivation
Abstract
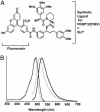
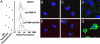
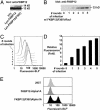
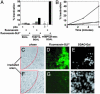
Chemical labeling of proteins inside of living cells can enable studies of the location, movement, and function of proteins in vivo. Here we demonstrate an approach for chemical labeling of proteins that uses the high-affinity interaction between an FKBP12 mutant (F36V) and a synthetic, engineered ligand (SLF'). A fluorescein conjugate to the engineered ligand (FL-SLF') retained binding to FKBP12(F36V) and possessed similar fluorescence properties as parental fluorescein. FL-SLF' labeled FKBP12(F36V) fusion proteins in live mammalian cells, and was used to monitor the subcellular localization of a membrane targeted FKBP12(F36V) construct. Chemical labeling of FKBP12(F36V) fusion proteins with FL-SLF' was readily detectable at low expression levels of the FKBP12(F36V) fusion, and the level of fluorescent staining with FL-SLF' was proportional to the FKBP12(F36V) expression level. This FL-SLF'-FKBP12(F36V) labeling technique was tested in fluorophore assisted laser inactivation (FALI), a light-mediated technique to rapidly inactivate fluorophore-labeled target proteins. FL-SLF' mediated FALI of a beta-galactosidase-FKBP12(F36V) fusion protein, causing rapid inactivation of >90% of enzyme activity upon irradiation in vitro. FL-SLF' also mediated FALI of a beta-galactosidase fusion expressed in living NIH 3T3 cells, where beta-galactosidase activity was reduced in 15 s. Thus, FL-SLF' can be used to monitor proteins in vivo and to target rapid, spatially and temporally defined inactivation of target proteins in living cells in a process that we call FK-FALI.
Figures

Similar articles
-
Fluorescent labeling of proteins in living cells using the FKBP12 (F36V) tag.Cytometry A. 2009 Mar;75(3):207-24. doi: 10.1002/cyto.a.20649. Cytometry A. 2009. PMID: 18837033
-
A bioorthogonal small-molecule-switch system for controlling protein function in live cells.Angew Chem Int Ed Engl. 2014 Sep 15;53(38):10049-55. doi: 10.1002/anie.201403463. Epub 2014 Jul 25. Angew Chem Int Ed Engl. 2014. PMID: 25065762
-
Fluorophore labeling of native FKBP12 by ligand-directed tosyl chemistry allows detection of its molecular interactions in vitro and in living cells.J Am Chem Soc. 2013 May 8;135(18):6782-5. doi: 10.1021/ja401956b. Epub 2013 Apr 26. J Am Chem Soc. 2013. PMID: 23611728
-
Bcl-2 and FKBP12 bind to IP3 and ryanodine receptors at overlapping sites: the complexity of protein-protein interactions for channel regulation.Biochem Soc Trans. 2015 Jun;43(3):396-404. doi: 10.1042/BST20140298. Biochem Soc Trans. 2015. PMID: 26009182 Review.
-
Chemical biology-based approaches on fluorescent labeling of proteins in live cells.Mol Biosyst. 2013 May;9(5):862-72. doi: 10.1039/c2mb25422k. Mol Biosyst. 2013. PMID: 23318293 Review.
Cited by
-
Histochemistry: live and in color.J Histochem Cytochem. 2011 Feb;59(2):139-45. doi: 10.1369/0022155410395760. J Histochem Cytochem. 2011. PMID: 21339179 Free PMC article. Review.
-
Chromophore-assisted laser inactivation in cell biology.Trends Cell Biol. 2008 Sep;18(9):443-50. doi: 10.1016/j.tcb.2008.07.001. Epub 2008 Aug 14. Trends Cell Biol. 2008. PMID: 18706812 Free PMC article. Review.
-
Development of a small peptide tag for covalent labeling of proteins.Bioconjug Chem. 2007 Jul-Aug;18(4):1318-24. doi: 10.1021/bc070080x. Epub 2007 Jun 30. Bioconjug Chem. 2007. PMID: 17602682 Free PMC article.
-
Development of benzothiazole 'click-on' fluorogenic dyes.Bioorg Med Chem Lett. 2011 Jan 1;21(1):320-3. doi: 10.1016/j.bmcl.2010.11.009. Epub 2010 Nov 5. Bioorg Med Chem Lett. 2011. PMID: 21111622 Free PMC article.
-
EGFR targeting PhosTACs as a dual inhibitory approach reveals differential downstream signaling.Sci Adv. 2024 Mar 29;10(13):eadj7251. doi: 10.1126/sciadv.adj7251. Epub 2024 Mar 27. Sci Adv. 2024. PMID: 38536914 Free PMC article.
References
-
- Tsien, R. Y. (1998) Annu. Rev. Biochem. 67, 509-544. - PubMed
-
- Zacharias, D. A., Violin, J. D., Newton, A. C. & Tsien, R. Y. (2002) Science 296, 913-916. - PubMed
-
- Griffin, B. A., Adams, S. R. & Tsien, R. Y. (1998) Science 281, 269-272. - PubMed
-
- Farinas, J. & Verkman, A. S. (1999) J. Biol. Chem. 274, 7603-7606. - PubMed
-
- Wu, M. M., Llopis, J., Adams, S., McCaffery, J. M., Kulomaa, M. S., Machen, T. E., Moore, H. P. & Tsien, R. Y. (2000) Chem. Biol. 7, 197-209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

