Plexin-B3 is a functional receptor for semaphorin 5A
- PMID: 15218527
- PMCID: PMC1299100
- DOI: 10.1038/sj.embor.7400189
Plexin-B3 is a functional receptor for semaphorin 5A
Abstract
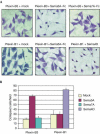
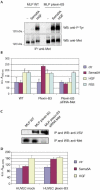
Semaphorins are a large family of molecular cues implicated in neural development and in a variety of functions outside the nervous system. Semaphorin 5A (Sema5A) is a transmembrane semaphorin, containing seven thrombospondin type-1 repeats, which was recently found to control axon guidance. Here we show that plexin-B3 is a high-affinity receptor specific for Sema5A. We further demonstrate that plexin-B3 activation by Sema5A mediates functional responses in plexin-B3-expressing cells (either fibroblasts, epithelial and primary endothelial cells). In addition, Sema5A can trigger the intracellular signalling of the hepatocyte growth factor/scatter factor receptor, Met, associated in a complex with plexin-B3. We thus conclude that Sema5A is able to elicit multiple functional responses through its receptor plexin-B3.
Figures

References
-
- Adams RH, Betz H, Puschel AW (1996) A novel class of murine semaphorins with homology to thrombospondin is differentially expressed during early embryogenesis. Mech Dev 57: 33–45 - PubMed
-
- Artigiani S, Comoglio PM, Tamagnone L (1999) Plexins, semaphorins, and scatter factor receptors: a common root for cell guidance signals? IUBMB Life 48: 477–482 - PubMed
-
- Artigiani S et al. (2003) Functional regulation of semaphorin receptors by proprotein convertases. J Biol Chem 278: 10094–10101 - PubMed
-
- Bahri SM, Chia W, Yang X (2001) Characterization and mutant analysis of the Drosophila sema 5c gene. Dev Dyn 221: 322–330 - PubMed
-
- Barberis D et al. (2004) Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J 18: 592–594 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

