Morphologic and genetic variability in the marine planktonic ciliate Laboea strobila Lohmann, 1908 (Ciliophora, Oligotrichia), with notes on its ontogenesis
- PMID: 15218695
- PMCID: PMC2859603
- DOI: 10.1111/j.1550-7408.2004.tb00567.x
Morphologic and genetic variability in the marine planktonic ciliate Laboea strobila Lohmann, 1908 (Ciliophora, Oligotrichia), with notes on its ontogenesis
Abstract
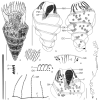
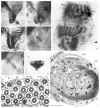
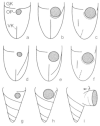
Laboea strobila Lohmann, 1908 is a conspicuous oligotrich ciliate in the marine plankton. In order to compare different populations, the morphology of specimens from the Mediterranean Sea, North Sea, and Irish Sea was investigated using live observation, protargol impregnation, and scanning electron microscopy. Furthermore, the PCR-amplified products of the SSrRNA gene from a monoclonal culture of L. strobila from the Mediterranean Sea were sequenced and aligned with sequences of other oligotrichs, including a population of L. strobila from the Atlantic coast of the USA. Finally, the data from the ecological literature were summarized and the cultivation methods were described. The SSrRNA gene sequences of the two distantly located L. strobila populations from the North Atlantic are identical. Likewise, the morphometrics of most populations so far investigated after protargol impregnation (i.e. from the North Atlantic) do not show obvious differences. In all computed phylogenetic trees, L. strobila groups with Strombidium species, forming a monophyletic taxon corresponding to the subclass Oligotrichia. These results are corroborated by the ontogenetic comparison. Since no type species was fixed for Laboea Lohmann, 1908, L. strobila was designated in the present paper.
Figures




References
-
- Aescht E. Catalogue of the generic names of ciliates (Protozoa, Ciliophora) Denisia. 2001;1:1–350.
-
- Agatha S. Oligotrichea (aloricate species) In: Costello MJ, Emblow CS, White R, editors. European Register of Marine Species. A Check-list of the Marine Species in Europe and a Bibliography of Guides to Their Identification. 2001. pp. 42–44. (Patrimoines naturels). 50.
-
- Agatha S. Morphology and ontogenesis of Novistrombidium apsheronicum nov. comb. and Strombidium arenicola (Protozoa, Ciliophora): a comparative light microscopical and SEM study. Europ. J. Protistol. 2003;39:245–266.
-
- Alekperov I. Kh., Asadullayeva ES. New and little-known ciliates (orders Nassulida-Oligotrichida) from the Caspian Sea Apsheronian coast. Communication 2. Zool. Zh. 1997;76:1411–1417. (in Russian with English summary)
-
- Alekperov IH, Mamajeva NV. Planktonic infusoria from the Chuckchee and the Bering Seas. Zool. Zh. 1992;71:5–14. (in Russian with English summary)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

