Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells
- PMID: 15220405
- PMCID: PMC434111
- DOI: 10.1128/JVI.78.14.7319-7328.2004
Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells
Abstract
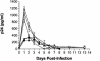
Although cells of monocytic lineage are the primary source of human immunodeficiency virus type 1 (HIV-1) in the brain, other cell types in the central nervous system, including astrocytes, can harbor a latent or persistent HIV-1 infection. In the present study, we examined whether immature, multipotential human brain-derived progenitor cells (nestin positive) are also permissive for infection. When exposed to IIIB and NL4-3 strains of HIV-1, progenitor cells and progenitor-derived astrocytes became infected, with peak p24 levels of 100 to 500 pg/ml at 3 to 6 days postinfection. After 10 days, virus production was undetectable but could be stimulated by the addition of tumor necrosis factor alpha (TNF-alpha). To bypass limitations to receptor entry, we compared the fate of infection in these cell populations by transfection with the infectious HIV-1 clone, pNL4-3. Again, transfected progenitors and astrocytes produced virus for 7 days but diminished to low levels beyond 8 days posttransfection. During the nonproductive phase, TNF-alpha stimulated virus production from progenitors as late as 5 weeks posttransfection. Astrocytes produced 5- to 20-fold more infectious virus (27 ng of p24/10(6) cells) than progenitors at the peak of 3 days posttransfection. Differentiation of infected progenitors toward an astrocyte phenotype increased virus production to levels consistent with infected astrocytes, suggesting a phenotypic difference in viral replication. Using this cell culture system of multipotential human brain-derived progenitor cells, we provide evidence that progenitor cells may be a reservoir for HIV-1 in the brains of AIDS patients.
Figures






Similar articles
-
Astrocyte differentiation selectively upregulates CCL2/monocyte chemoattractant protein-1 in cultured human brain-derived progenitor cells.Glia. 2006 Jan 1;53(1):81-91. doi: 10.1002/glia.20261. Glia. 2006. PMID: 16206198
-
Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection.J Hum Virol. 2000 Nov-Dec;3(6):324-34. J Hum Virol. 2000. PMID: 11100913
-
Long-term HIV-1 infection of neural progenitor populations.AIDS. 2007 Nov 12;21(17):2271-81. doi: 10.1097/QAD.0b013e3282f12f27. AIDS. 2007. PMID: 18090275
-
Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus.Virus Res. 2005 Aug;111(2):194-213. doi: 10.1016/j.virusres.2005.04.009. Virus Res. 2005. PMID: 15885841 Review.
-
Stages of restricted HIV-1 infection in astrocyte cultures derived from human fetal brain tissue.J Neurovirol. 2000 May;6 Suppl 1:S90-4. J Neurovirol. 2000. PMID: 10871771 Review.
Cited by
-
Enigma of HIV-1 latent infection in astrocytes: an in-vitro study using protein kinase C agonist as a latency reversing agent.Microbes Infect. 2015 Sep;17(9):651-9. doi: 10.1016/j.micinf.2015.05.006. Epub 2015 Jun 2. Microbes Infect. 2015. PMID: 26043820 Free PMC article.
-
Gamma interferon primes productive human immunodeficiency virus infection in astrocytes.J Virol. 2006 Jan;80(1):541-4. doi: 10.1128/JVI.80.1.541-544.2006. J Virol. 2006. PMID: 16352578 Free PMC article.
-
Adult neurogenic deficits in HIV-1 Tg26 transgenic mice.J Neuroinflammation. 2018 Oct 12;15(1):287. doi: 10.1186/s12974-018-1322-2. J Neuroinflammation. 2018. PMID: 30314515 Free PMC article.
-
Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme.Br J Pharmacol. 2015 Oct;172(19):4603-14. doi: 10.1111/bph.12657. Epub 2015 Aug 21. Br J Pharmacol. 2015. PMID: 24571443 Free PMC article.
-
A fully human antibody to gp41 selectively eliminates HIV-infected cells that transmigrated across a model human blood brain barrier.AIDS. 2016 Feb 20;30(4):563-72. doi: 10.1097/QAD.0000000000000968. AIDS. 2016. PMID: 26595540 Free PMC article.
References
-
- An, S. F., M. Groves, B. Giometto, A. A. Beckett, and F. Scaravilli. 1999. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. 98:481-487. - PubMed
-
- Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573-585. - PubMed
-
- Bell, J. E. 1998. The neuropathology of adult HIV infection. Rev. Neurol. 154:816-829. - PubMed
-
- Brack-Werner, R. 1999. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

