The N-terminal 85 amino acids of the barley stripe mosaic virus gammab pathogenesis protein contain three zinc-binding motifs
- PMID: 15220411
- PMCID: PMC434125
- DOI: 10.1128/JVI.78.14.7379-7391.2004
The N-terminal 85 amino acids of the barley stripe mosaic virus gammab pathogenesis protein contain three zinc-binding motifs
Abstract
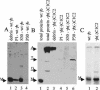
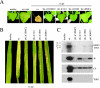
Barley stripe mosaic virus RNAgamma encodes gammab, a cysteine-rich protein that affects pathogenesis. Nine of the eleven cysteines are concentrated in two clusters, designated C1 (residues 1 to 23) and C2 (residues 60 to 85), that are arranged in zinc finger-like motifs. A basic motif (BM) rich in lysine and arginine (residues 19 to 47) resides between the C1 and C2 clusters. We have demonstrated that gammab binds zinc and that the C1, BM, and C2 motifs have independent zinc-binding activities. To evaluate the requirements for binding, mutations were introduced into each region. Cysteine residues at positions 7, 9, 10, 19, and 23 in the C1 motif were replaced with serines. In the BM, asparagines were substituted for lysines at positions 26 and 35, glutamine for arginine at position 25, and glycines for arginines at positions 33 and 36. The C2 mutations included cysteine replacements with serines at positions 60, 64, 71, and 81, and a histidine-to-leucine change at position 85. These mutations destroyed zinc-binding activity in each of the isolated motifs. gammab derivatives containing mutations in only two of the motifs retained the ability to bind zinc, whereas a gammab derivative containing mutations inactivating all three motifs destroyed the ability to bind zinc. Plants inoculated with transcripts containing combinations of the C1, BM, and C2 mutations elicited a "null" phenotype in barley characteristic of gammab deletion mutants and also delayed the appearance and reduced the size of local lesions in Chenopodium amaranticolor. These results show that zinc binding of each of the motifs is critical for the biological activity of gammab.
Figures





Similar articles
-
The C-terminal region of the Barley stripe mosaic virusgammab protein participates in homologous interactions and is required for suppression of RNA silencing.Mol Plant Pathol. 2004 Sep 1;5(5):465-81. doi: 10.1111/j.1364-3703.2004.00246.x. Mol Plant Pathol. 2004. PMID: 20565621
-
Mapping of the seed transmission determinants of barley stripe mosaic virus.Mol Plant Microbe Interact. 1995 Nov-Dec;8(6):906-15. doi: 10.1094/mpmi-8-0906. Mol Plant Microbe Interact. 1995. PMID: 8664501
-
RNA-binding activities of barley stripe mosaic virus gamma b fusion proteins.J Gen Virol. 1996 May;77 ( Pt 5):879-88. doi: 10.1099/0022-1317-77-5-879. J Gen Virol. 1996. PMID: 8609484
-
The barley stripe mosaic virus gamma b gene encodes a multifunctional cysteine-rich protein that affects pathogenesis.Plant Cell. 1994 Nov;6(11):1593-606. doi: 10.1105/tpc.6.11.1593. Plant Cell. 1994. PMID: 7827493 Free PMC article.
-
Zinc-binding cysteines: diverse functions and structural motifs.Biomolecules. 2014 Apr 17;4(2):419-34. doi: 10.3390/biom4020419. Biomolecules. 2014. PMID: 24970223 Free PMC article. Review.
Cited by
-
The Barley stripe mosaic virus γb protein promotes viral cell-to-cell movement by enhancing ATPase-mediated assembly of ribonucleoprotein movement complexes.PLoS Pathog. 2020 Jul 30;16(7):e1008709. doi: 10.1371/journal.ppat.1008709. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32730331 Free PMC article.
-
Phosphorylation of plant virus proteins: Analysis methods and biological functions.Front Microbiol. 2022 Jul 26;13:935735. doi: 10.3389/fmicb.2022.935735. eCollection 2022. Front Microbiol. 2022. PMID: 35958157 Free PMC article. Review.
-
Advances in understanding multifunctionality of Barley stripe mosaic virus γb protein.PLoS Pathog. 2025 Jul 2;21(7):e1013299. doi: 10.1371/journal.ppat.1013299. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40601703 Free PMC article. Review.
-
Identification of an attenuated barley stripe mosaic virus for the virus-induced gene silencing of pathogenesis-related wheat genes.Plant Methods. 2016 Feb 2;12:12. doi: 10.1186/s13007-016-0112-z. eCollection 2016. Plant Methods. 2016. PMID: 26839581 Free PMC article.
-
Barley stripe mosaic virus γb protein disrupts chloroplast antioxidant defenses to optimize viral replication.EMBO J. 2021 Aug 16;40(16):e107660. doi: 10.15252/embj.2021107660. Epub 2021 Jul 13. EMBO J. 2021. PMID: 34254679 Free PMC article.
References
-
- Auld, D. S. 2001. Zinc coordination sphere in biochemical zinc sites. Biometals 14:271-313. - PubMed
-
- Berg, J. M., and H. A. Godwin. 1997. Lessons from zinc-binding peptides. Annu. Rev. Biophys. Biomol. Struct. 26:357-371. - PubMed
-
- Donald, R. G. K., and A. O. Jackson. 1996. RNA-binding activities of barley stripe mosaic virus γb fusion proteins. J. Gen. Virol. 77:879-888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

