Structure of D-63 from sulfolobus spindle-shaped virus 1: surface properties of the dimeric four-helix bundle suggest an adaptor protein function
- PMID: 15220417
- PMCID: PMC434073
- DOI: 10.1128/JVI.78.14.7438-7442.2004
Structure of D-63 from sulfolobus spindle-shaped virus 1: surface properties of the dimeric four-helix bundle suggest an adaptor protein function
Abstract
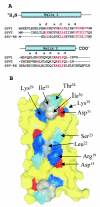
Sulfolobus spindle-shaped virus 1 (SSV1) and its fusellovirus homologues can be found in many acidic (pH <or= 4.0) hot springs (>or=70 degrees C) around the world. SSV1 contains a 15.5-kb double-stranded DNA genome that encodes 34 proteins with greater than 50 amino acids. A site-specific integrase and a DnaA-like protein have been previously identified by sequence homology, and three structural proteins have been isolated from purified virus and identified by N-terminal sequencing (VP1, VP2, and VP3). The functions of the remaining 29 proteins are currently unknown. To assign functions to these proteins, we have initiated biochemical and structural studies on the SSV1 proteome. Here we report the structure of SSV1 D-63. The structure reveals a helix-turn-helix motif that dimerizes to form an antiparallel four-helix bundle. Mapping residues conserved among three fusellovirus isolates onto the structure shows that one face of the rod-shaped molecule is highly conserved. This conserved surface spans the dimer axis and thus exhibits 2-fold symmetry. Two smaller conserved patches, also related by 2-fold symmetry, are found on the opposite face of the molecule. All of these conserved surfaces are devoid of clefts or pockets typically used to bind small molecules, suggesting that D-63 may function as an adaptor protein in macromolecular assembly.
Figures

Similar articles
-
Extreme Mutation Tolerance: Nearly Half of the Archaeal Fusellovirus Sulfolobus Spindle-Shaped Virus 1 Genes Are Not Required for Virus Function, Including the Minor Capsid Protein Gene vp3.J Virol. 2017 Apr 28;91(10):e02406-16. doi: 10.1128/JVI.02406-16. Print 2017 May 15. J Virol. 2017. PMID: 28148789 Free PMC article.
-
Sulfolobus Spindle-Shaped Virus 1 Contains Glycosylated Capsid Proteins, a Cellular Chromatin Protein, and Host-Derived Lipids.J Virol. 2015 Nov;89(22):11681-91. doi: 10.1128/JVI.02270-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355093 Free PMC article.
-
Cysteine usage in Sulfolobus spindle-shaped virus 1 and extension to hyperthermophilic viruses in general.Virology. 2008 Jul 5;376(2):270-8. doi: 10.1016/j.virol.2008.03.026. Epub 2008 May 8. Virology. 2008. PMID: 18471851
-
Molecular biology of fuselloviruses and their satellites.Extremophiles. 2014 May;18(3):473-89. doi: 10.1007/s00792-014-0634-0. Epub 2014 Feb 23. Extremophiles. 2014. PMID: 24562787 Review.
-
Genomics, Transcriptomics, and Proteomics of SSV1 and Related Fusellovirus: A Minireview.Viruses. 2022 Sep 20;14(10):2082. doi: 10.3390/v14102082. Viruses. 2022. PMID: 36298638 Free PMC article. Review.
Cited by
-
Acidianus Tailed Spindle Virus: a New Archaeal Large Tailed Spindle Virus Discovered by Culture-Independent Methods.J Virol. 2016 Jan 13;90(7):3458-68. doi: 10.1128/JVI.03098-15. J Virol. 2016. PMID: 26763997 Free PMC article.
-
The thermo- and acido-stable ORF-99 from the archaeal virus AFV1.Protein Sci. 2009 Jun;18(6):1316-20. doi: 10.1002/pro.122. Protein Sci. 2009. PMID: 19472363 Free PMC article.
-
Extreme Mutation Tolerance: Nearly Half of the Archaeal Fusellovirus Sulfolobus Spindle-Shaped Virus 1 Genes Are Not Required for Virus Function, Including the Minor Capsid Protein Gene vp3.J Virol. 2017 Apr 28;91(10):e02406-16. doi: 10.1128/JVI.02406-16. Print 2017 May 15. J Virol. 2017. PMID: 28148789 Free PMC article.
-
The structure of the CRISPR-associated protein Csa3 provides insight into the regulation of the CRISPR/Cas system.J Mol Biol. 2011 Jan 28;405(4):939-55. doi: 10.1016/j.jmb.2010.11.019. Epub 2010 Nov 18. J Mol Biol. 2011. PMID: 21093452 Free PMC article.
-
Plasmids and viruses of the thermoacidophilic crenarchaeote Sulfolobus.Extremophiles. 2006 Feb;10(1):17-28. doi: 10.1007/s00792-005-0492-x. Epub 2006 Jan 6. Extremophiles. 2006. PMID: 16397749 Review.
References
-
- Bailey, S. 1994. The CCP4 suite—programs for protein crystallography. Acta Crystallogr. D 50:760-763. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Cowtan, K. 1994. DM: an automated procedure for phase improvement by density modification. Joint CCP4 ESF-EACBM Newsl. Protein Crystallogr. 31:34-38.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

