Short interfering RNA accumulation correlates with host recovery in DNA virus-infected hosts, and gene silencing targets specific viral sequences
- PMID: 15220420
- PMCID: PMC434130
- DOI: 10.1128/JVI.78.14.7465-7477.2004
Short interfering RNA accumulation correlates with host recovery in DNA virus-infected hosts, and gene silencing targets specific viral sequences
Erratum in
- J Virol. 2006 Jan;80(2):1064. Pita, Justin [added]
Abstract
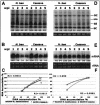
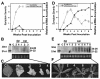
Viruses are both inducers and targets of posttranscriptional gene silencing (PTGS), a natural defense mechanism in plants. Here we report molecular evidence of the ability of single-stranded DNA (ssDNA) viruses to induce PTGS in infected plants irrespective of the severity of or recovery from the symptoms. Our results reveal that five distinct species of cassava-infecting geminiviruses were capable of triggering PTGS by producing two classes of virus-specific short interfering RNAs (siRNAs) of 21 to 26 nucleotides in two plant hosts, tobacco (Nicotiana benthamiana) and cassava (Manihot esculenta, Crantz). However, the efficacy of virus-induced PTGS varied depending on the intrinsic features of the virus and its interaction with the plant host. We found that symptom recovery over time in plants infected with the isolates of African cassava mosaic virus (ACMV-[CM]) or Sri Lankan cassava mosaic virus was associated with a much higher level of virus-derived siRNA accumulation compared to plants infected with viruses that do not show symptom recovery. Furthermore, we determined that the C terminus of AC1 that overlaps with the N terminus of AC2 early viral genes involved in virus replication were the primary targets for ACMV-[CM]-induced PTGS, whereas the C terminus of BC1 was targeted for the East African cassava mosaic Cameroon virus. In addition, our results reveal the possibility for double-stranded RNA formation during transcription in ssDNA viruses, which explains in part how these viruses can trigger PTGS in plants.
Figures





Similar articles
-
Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing.J Virol. 2004 Sep;78(17):9487-98. doi: 10.1128/JVI.78.17.9487-9498.2004. J Virol. 2004. PMID: 15308741 Free PMC article.
-
Evidence of synergism between African cassava mosaic virus and a new double-recombinant geminivirus infecting cassava in Cameroon.J Gen Virol. 2000 Jan;81(Pt 1):287-97. doi: 10.1099/0022-1317-81-1-287. J Gen Virol. 2000. PMID: 10640569
-
Effect of temperature on geminivirus-induced RNA silencing in plants.Plant Physiol. 2005 Aug;138(4):1828-41. doi: 10.1104/pp.105.066563. Epub 2005 Jul 22. Plant Physiol. 2005. PMID: 16040661 Free PMC article.
-
Geminiviruses and RNA silencing.Trends Plant Sci. 2005 Mar;10(3):144-51. doi: 10.1016/j.tplants.2005.01.005. Trends Plant Sci. 2005. PMID: 15749473 Review.
-
How can plant DNA viruses evade siRNA-directed DNA methylation and silencing?Int J Mol Sci. 2013 Jul 24;14(8):15233-59. doi: 10.3390/ijms140815233. Int J Mol Sci. 2013. PMID: 23887650 Free PMC article. Review.
Cited by
-
Cassava mosaic geminiviruses: actual knowledge and perspectives.Mol Plant Pathol. 2009 Sep;10(5):685-701. doi: 10.1111/j.1364-3703.2009.00559.x. Mol Plant Pathol. 2009. PMID: 19694957 Free PMC article. Review.
-
BmNPV p35 Reduces the Accumulation of Virus-Derived siRNAs and Hinders the Function of siRNAs to Facilitate Viral Infection.Front Immunol. 2022 Feb 18;13:845268. doi: 10.3389/fimmu.2022.845268. eCollection 2022. Front Immunol. 2022. PMID: 35251046 Free PMC article.
-
MicroRNA-binding viral protein interferes with Arabidopsis development.Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10381-6. doi: 10.1073/pnas.0504439102. Epub 2005 Jul 8. Proc Natl Acad Sci U S A. 2005. PMID: 16006510 Free PMC article.
-
Small RNAs tackle large viruses: RNA interference-based antiviral defense against DNA viruses in insects.Fly (Austin). 2013 Oct-Dec;7(4):216-23. doi: 10.4161/fly.25708. Epub 2013 Aug 23. Fly (Austin). 2013. PMID: 23974177 Free PMC article.
-
Generation of marker-free transgenic plants concurrently resistant to a DNA geminivirus and a RNA tospovirus.Sci Rep. 2014 Jul 17;4:5717. doi: 10.1038/srep05717. Sci Rep. 2014. PMID: 25030413 Free PMC article.
References
-
- Al-Kaff, N. S., S. N. Covey, M. M. Kreike, A. M. Page, R. Pinder, and P. J. Dale. 1998. Transcriptional and posttranscriptional plant gene silencing in response to a pathogen. Science 279:2113-2115. - PubMed
-
- Baulcombe, D. C. 1996. RNA as a target and an initiator of posttranscriptional gene silencing in transgenic plants. Plant Mol. Biol. 32:79-88. - PubMed
-
- Bernstein, E., A. A. Caudy, S. M. Hammond, and G. J. Hannon. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363-366. - PubMed
-
- Cock, J. H. 1985. Cassava: new potential for a neglected crop. Westview Press, Boulder, Colo.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

