Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys
- PMID: 15220422
- PMCID: PMC434100
- DOI: 10.1128/JVI.78.14.7490-7497.2004
Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys
Abstract
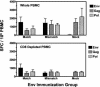
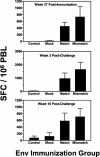
Because a strategy to elicit broadly neutralizing anti-human immunodeficiency virus type 1 (HIV-1) antibodies has not yet been found, the role of an Env immunogen in HIV-1 vaccine candidates remains undefined. We sought to determine whether an HIV-1 Env immunogen genetically disparate from the Env of the challenge virus can contribute to protective immunity. We vaccinated Indian-origin rhesus monkeys with Gag-Pol-Nef immunogens, alone or in combination with Env immunogens that were either matched or mismatched with the challenge virus. These animals were then challenged with a pathogenic simian-human immunodeficiency virus. The vaccine regimen included a plasmid DNA prime and replication-defective adenoviral vector boost. Vaccine regimens that included the matched or mismatched Env immunogens conferred better protection against CD4(+) T-lymphocyte loss than that seen with comparable regimens that did not include Env immunogens. This increment in protective immunity was associated with anamnestic Env-specific cellular immunity that developed in the early days following viral challenge. These data suggest that T-lymphocyte immunity to Env can broaden the protective cellular immune response to HIV despite significant sequence diversity of the strains of the Env immunogens and can contribute to immune protection in this AIDS vaccine model.
Figures





Similar articles
-
Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys.J Virol. 2005 Oct;79(19):12321-31. doi: 10.1128/JVI.79.19.12321-12331.2005. J Virol. 2005. PMID: 16160159 Free PMC article.
-
Chimeric adenovirus type 5/35 vector encoding SIV gag and HIV env genes affords protective immunity against the simian/human immunodeficiency virus in monkeys.Virology. 2007 Oct 25;367(2):390-7. doi: 10.1016/j.virol.2007.06.012. Epub 2007 Jul 12. Virology. 2007. PMID: 17628628
-
Immunization with HIV-1 SF162-derived Envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection.Virology. 2006 Jun 5;349(2):276-89. doi: 10.1016/j.virol.2006.01.043. Epub 2006 Mar 9. Virology. 2006. PMID: 16527321
-
Current approaches to vaccination against human immunodeficiency viruses.Allergol Immunopathol (Madr). 1991 Jan-Feb;19(1):11-3. Allergol Immunopathol (Madr). 1991. PMID: 1719790 Review. No abstract available.
-
Simian retrovirus vaccines: simian retrovirus and simian immunodeficiency lentivirus.AIDS Res Hum Retroviruses. 1996 Mar 20;12(5):399-401. doi: 10.1089/aid.1996.12.399. AIDS Res Hum Retroviruses. 1996. PMID: 8882318 Review. No abstract available.
Cited by
-
Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques.J Virol. 2010 Jul;84(14):7161-73. doi: 10.1128/JVI.00410-10. Epub 2010 May 5. J Virol. 2010. PMID: 20444898 Free PMC article.
-
Memory CD4+ T-lymphocyte loss and dysfunction during primary simian immunodeficiency virus infection.J Virol. 2007 Aug;81(15):8009-15. doi: 10.1128/JVI.00482-07. Epub 2007 May 23. J Virol. 2007. PMID: 17522197 Free PMC article.
-
HIV DNA Vaccine: Stepwise Improvements Make a Difference.Vaccines (Basel). 2014 May 14;2(2):354-79. doi: 10.3390/vaccines2020354. Vaccines (Basel). 2014. PMID: 26344623 Free PMC article. Review.
-
Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins.Virology. 2007 Mar 30;360(1):218-34. doi: 10.1016/j.virol.2006.10.017. Epub 2006 Nov 13. Virology. 2007. PMID: 17097711 Free PMC article.
-
The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert.Clin Vaccine Immunol. 2010 Nov;17(11):1687-94. doi: 10.1128/CVI.00311-10. Epub 2010 Sep 8. Clin Vaccine Immunol. 2010. PMID: 20826614 Free PMC article.
References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davis, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. H. I. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian human immunodeficiency virus. J. Virol. 73:10199-10207. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

