Cellular and viral requirements for rapid endocytic entry of herpes simplex virus
- PMID: 15220424
- PMCID: PMC434080
- DOI: 10.1128/JVI.78.14.7508-7517.2004
Cellular and viral requirements for rapid endocytic entry of herpes simplex virus
Abstract
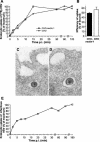
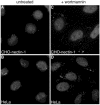
It was recently demonstrated that herpes simplex virus (HSV) successfully infects Chinese hamster ovary (CHO) cells expressing glycoprotein D (gD) receptors and HeLa cells by an endocytic mechanism (A. V. Nicola, A. M. McEvoy, and S. E. Straus, J. Virol. 77:5324-5332, 2003). Here we define cellular and viral requirements of this pathway. Uptake of intact, enveloped HSV from the cell surface into endocytic vesicles was rapid (t(1/2) of 8 to 9 min) and independent of the known cell surface gD receptors. Following uptake from the surface, recovery of intracellular, infectious virions increased steadily up to 20 min postinfection (p.i.), which corresponds to accumulation of enveloped virus in intracellular compartments. There was a sharp decline in recovery by 30 min p.i., suggesting loss of the virus envelope as a result of capsid penetration from endocytic organelles into the cytosol. In the absence of gD receptors, endocytosed virions did not successfully penetrate into the cytosol but were instead transported to lysosomes for degradation. Inhibitors of phosphatidylinositol (PI) 3-kinase, such as wortmannin, blocked transport of incoming HSV to the nuclear periphery and virus-induced gene expression but had no effect on virus binding or uptake. This suggests a role for PI 3-kinase activity in trafficking of HSV through the cytosol. Viruses that lack viral glycoproteins gB, gD, or gH-gL were defective in transport to the nucleus and had reduced infectivity. Thus, similar to entry via direct penetration at the cell surface, HSV entry into cells by wortmannin-sensitive endocytosis is efficient, involves rapid cellular uptake of viral particles, and requires gB, gD, and gH-gL.
Figures

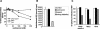
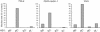

Similar articles
-
Structure-function analysis of herpes simplex virus glycoprotein B with fusion-from-without activity.Virology. 2008 Dec 20;382(2):207-16. doi: 10.1016/j.virol.2008.09.015. Epub 2008 Oct 23. Virology. 2008. PMID: 18950828
-
Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells.Virol J. 2006 Dec 27;3:105. doi: 10.1186/1743-422X-3-105. Virol J. 2006. PMID: 17192179 Free PMC article.
-
Use of chimeric nectin-1(HveC)-related receptors to demonstrate that ability to bind alphaherpesvirus gD is not necessarily sufficient for viral entry.Virology. 2001 Jul 5;285(2):366-75. doi: 10.1006/viro.2001.0989. Virology. 2001. PMID: 11437670
-
The role of herpes simplex virus glycoproteins in the virus replication cycle.Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review.
-
Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry.Virology. 2006 Jan 5;344(1):17-24. doi: 10.1016/j.virol.2005.09.016. Virology. 2006. PMID: 16364731 Review.
Cited by
-
Herpesvirus transport to the nervous system and back again.Annu Rev Microbiol. 2012;66:153-76. doi: 10.1146/annurev-micro-092611-150051. Epub 2012 Jun 15. Annu Rev Microbiol. 2012. PMID: 22726218 Free PMC article. Review.
-
HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread.PLoS One. 2014 Feb 21;9(2):e88803. doi: 10.1371/journal.pone.0088803. eCollection 2014. PLoS One. 2014. PMID: 24586397 Free PMC article.
-
Go go gadget glycoprotein!: HSV-1 draws on its sizeable glycoprotein tool kit to customize its diverse entry routes.PLoS Pathog. 2019 May 9;15(5):e1007660. doi: 10.1371/journal.ppat.1007660. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31071197 Free PMC article. No abstract available.
-
Entry mechanisms of herpes simplex virus 1 into murine epidermis: involvement of nectin-1 and herpesvirus entry mediator as cellular receptors.J Virol. 2015 Jan;89(1):262-74. doi: 10.1128/JVI.02917-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320325 Free PMC article.
-
The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside.Virology. 2015 May;479-480:568-77. doi: 10.1016/j.virol.2015.02.040. Epub 2015 Mar 20. Virology. 2015. PMID: 25798530 Free PMC article. Review.
References
-
- Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa B kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

