Determinants of human immunodeficiency virus type 1 baseline susceptibility to the fusion inhibitors enfuvirtide and T-649 reside outside the peptide interaction site
- PMID: 15220433
- PMCID: PMC434069
- DOI: 10.1128/JVI.78.14.7582-7589.2004
Determinants of human immunodeficiency virus type 1 baseline susceptibility to the fusion inhibitors enfuvirtide and T-649 reside outside the peptide interaction site
Abstract
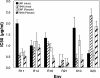
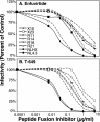
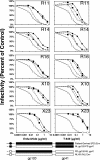
The peptide fusion inhibitor (PFI) enfuvirtide is the first of a new class of entry inhibitors to receive FDA approval. We previously determined the susceptibility of 55 PFI-naïve-patient isolates to enfuvirtide and a second peptide inhibitor, T-649. Seven of the 55 viral isolates were insusceptible to enfuvirtide, T-649, or both inhibitors in the absence of prior exposure. To determine the molecular basis of the insusceptible phenotypes, we PCR amplified and cloned five PFI-insusceptible and one PFI-susceptible, full-length, biologically functional env genes and characterized viruses pseudotyped with the Env proteins in a single-round drug sensitivity assay. Overall, the mean 50% inhibitory concentrations of enfuvirtide and T-649 for the PFI-insusceptible Env pseudotypes were 1.4 to 1.7 log(10) and 1.2 to 1.8 log(10) greater, respectively, than those for a PFI-susceptible lab strain, NLHX; however, all of the PFI-insusceptible Env proteins conserved the sequence of a critical enfuvirtide interaction site (residues 36 to 38 of gp41, GIV) in HR-1. In contrast, multiple amino acid changes were observed C-terminal to HR-1, many of which were located in regions of HR-2 corresponding to the PFI. Nevertheless, peptides based on patient-derived HR-2 sequences were not more potent inhibitors than enfuvirtide or T-649, arguing that the basis of PFI susceptibility is not a higher-affinity, competitive HR-1/HR-2 interaction. These results demonstrate that regions of Env outside the enfuvirtide interaction site can significantly impact the PFI susceptibility of patient-derived Env, even prior to drug exposure. We hypothesize that both gp120 gene- and gp41 gene-encoded determinants that minimize the window of opportunity for PFI to bind provide a growth advantage and possibly a predisposition to resistance to this new class of drugs in vivo.
Figures

Similar articles
-
Role of the envelope genetic context in the development of enfuvirtide resistance in human immunodeficiency virus type 1-infected patients.J Virol. 2006 Sep;80(17):8807-19. doi: 10.1128/JVI.02706-05. J Virol. 2006. PMID: 16912327 Free PMC article.
-
Impact of human immunodeficiency virus type 1 gp41 amino acid substitutions selected during enfuvirtide treatment on gp41 binding and antiviral potency of enfuvirtide in vitro.J Virol. 2005 Oct;79(19):12447-54. doi: 10.1128/JVI.79.19.12447-12454.2005. J Virol. 2005. PMID: 16160172 Free PMC article.
-
Resistance and replicative capacity of HIV-1 strains selected in vivo by long-term enfuvirtide treatment.New Microbiol. 2004 Apr;27(2 Suppl 1):51-61. New Microbiol. 2004. PMID: 15646065
-
Resistance to enfuvirtide, the first HIV fusion inhibitor.J Antimicrob Chemother. 2004 Aug;54(2):333-40. doi: 10.1093/jac/dkh330. Epub 2004 Jul 1. J Antimicrob Chemother. 2004. PMID: 15231762 Review.
-
HIV entry inhibitors: mechanisms of action and resistance pathways.J Antimicrob Chemother. 2006 Apr;57(4):619-27. doi: 10.1093/jac/dkl027. Epub 2006 Feb 7. J Antimicrob Chemother. 2006. PMID: 16464888 Review.
Cited by
-
Expanded tropism and altered activation of a retroviral glycoprotein resistant to an entry inhibitor peptide.J Virol. 2006 Jan;80(1):353-9. doi: 10.1128/JVI.80.1.353-359.2006. J Virol. 2006. PMID: 16352560 Free PMC article.
-
Functional diversity of HIV-1 envelope proteins expressed by contemporaneous plasma viruses.Retrovirology. 2008 Feb 29;5:23. doi: 10.1186/1742-4690-5-23. Retrovirology. 2008. PMID: 18312646 Free PMC article.
-
Role of the envelope genetic context in the development of enfuvirtide resistance in human immunodeficiency virus type 1-infected patients.J Virol. 2006 Sep;80(17):8807-19. doi: 10.1128/JVI.02706-05. J Virol. 2006. PMID: 16912327 Free PMC article.
-
Appreciating HIV type 1 diversity: subtype differences in Env.AIDS Res Hum Retroviruses. 2009 Mar;25(3):237-48. doi: 10.1089/aid.2008.0219. AIDS Res Hum Retroviruses. 2009. PMID: 19327047 Free PMC article.
-
Donor and recipient envs from heterosexual human immunodeficiency virus subtype C transmission pairs require high receptor levels for entry.J Virol. 2010 Apr;84(8):4100-4. doi: 10.1128/JVI.02068-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147398 Free PMC article.
References
-
- Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. - PubMed
-
- Chou, P. Y., and G. D. Fasman. 1978. Empirical predictions of protein conformation. Annu. Rev. Biochem. 47:251-276. - PubMed
-
- Cole, J. L., and V. M. Garsky. 2001. Thermodynamics of peptide inhibitor binding to HIV-1 gp41. Biochemistry 40:5633-5641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

