Immunostimulant patch enhances immune responses to influenza virus vaccine in aged mice
- PMID: 15220436
- PMCID: PMC434114
- DOI: 10.1128/JVI.78.14.7610-7618.2004
Immunostimulant patch enhances immune responses to influenza virus vaccine in aged mice
Abstract
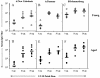
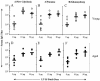
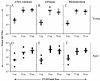
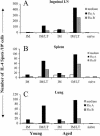
Improvement in the immune response to influenza virus vaccination in the elderly represents the primary unmet need in influenza virus vaccination. We have shown that topical application of immunostimulating (IS) patches containing heat-labile enterotoxin of Escherichia coli (LT) enhances immune responses to injected vaccines. We extend these findings and show that LT-IS patch application enhances the antibody responses to influenza virus vaccination in both young and aged mice. LT-IS patches markedly increased influenza virus-specific immunoglobulin G (IgG), hemagglutination inhibition antibody, mucosal antibody, and T-cell responses. The magnitude of the immune responses in aged mice receiving an LT-IS patch was equivalent to or greater than that of the immune responses in young mice given vaccine alone. These results suggest that addition of an LT-IS patch may compensate for the deficient immune function seen in the aged in response to influenza virus vaccination. Therefore, use of an LT-IS patch could be a new, safe, and simple immunization strategy that may significantly improve the outcome of influenza virus vaccination in the elderly.
Figures



Similar articles
-
Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells.J Virol. 2003 May;77(9):5218-25. doi: 10.1128/jvi.77.9.5218-5225.2003. J Virol. 2003. PMID: 12692224 Free PMC article.
-
Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine.J Infect Dis. 1997 Feb;175(2):352-63. doi: 10.1093/infdis/175.2.352. J Infect Dis. 1997. PMID: 9203656 Clinical Trial.
-
Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch.Vaccine. 2005 Jan 4;23(7):946-50. doi: 10.1016/j.vaccine.2004.06.036. Vaccine. 2005. PMID: 15603897
-
A proposal for safety standards for human use of cholera toxin (or Escherichia coli heat-labile enterotoxin) derivatives as an adjuvant of nasal inactivated influenza vaccine.Jpn J Infect Dis. 2000 Jun;53(3):98-106. Jpn J Infect Dis. 2000. PMID: 10957706 Review.
-
Transcutaneous immunization with heat-labile enterotoxin: development of a needle-free vaccine patch.Expert Rev Vaccines. 2007 Oct;6(5):809-19. doi: 10.1586/14760584.6.5.809. Expert Rev Vaccines. 2007. PMID: 17931160 Review.
Cited by
-
Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: a histologic and immunophenotypic analysis.J Transl Med. 2010 Aug 20;8:79. doi: 10.1186/1479-5876-8-79. J Transl Med. 2010. PMID: 20727190 Free PMC article. Clinical Trial.
-
NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge.J Virol. 2012 Oct;86(19):10293-301. doi: 10.1128/JVI.01131-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787224 Free PMC article.
-
Skin immunization with influenza vaccines.Curr Top Microbiol Immunol. 2015;386:343-69. doi: 10.1007/82_2014_407. Curr Top Microbiol Immunol. 2015. PMID: 25038939 Free PMC article. Review.
-
Immunostimulant adjuvant patch enhances humoral and cellular immune responses to DNA immunization.DNA Cell Biol. 2008 Jan;27(1):19-24. doi: 10.1089/dna.2007.0639. DNA Cell Biol. 2008. PMID: 17961074 Free PMC article.
-
Topical CpG adjuvantation of a protein-based vaccine induces protective immunity to Listeria monocytogenes.Clin Vaccine Immunol. 2014 Mar;21(3):329-39. doi: 10.1128/CVI.00734-13. Epub 2014 Jan 3. Clin Vaccine Immunol. 2014. PMID: 24391136 Free PMC article.
References
-
- Barker, W. H., and J. P. Mullooly. 1980. Impact of epidemic type A influenza in a defined adult population. Am. J. Epidemiol. 112:798-811. - PubMed
-
- Cella, M., A. Engering, V. Pinet, J. Pieters, and A. Lanzavecchia. 1997. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388:782-787. - PubMed
-
- Crocetti, E., S. Arniani, F. Bordoni, G. Maciocco, M. Zappa, and E. Buiatti. 2001. Effectiveness of influenza vaccination in the elderly in a community in Italy. Eur. J. Epidemiol. 17:163-168. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

