A probable Na+(K+)/H+ exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana
- PMID: 15220473
- PMCID: PMC454189
- DOI: 10.1073/pnas.0403709101
A probable Na+(K+)/H+ exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana
Abstract
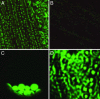
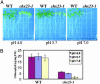
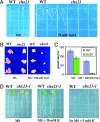
Electroneutral monovalent cation/proton antiport across the chloroplast envelope has been shown previously to have an important regulatory effect on stromal pH and thereby on photosynthetic carbon reduction. Here we report that an Arabidopsis nuclear gene, AtCHX23, encodes a putative Na(+)(K(+))/H(+) exchanger and functions in the adjustment of pH in the cytosol and possibly in maintaining a high pH level in the chloroplast stroma. The AtCHX23 protein is localized in the chloroplast envelope. Plastids from chx23 mutants had straight thylakoids but lacked grana lamellae. chx23 mutant leaves were pale yellow and had a much reduced chlorophyll content. The chlorophyll content of chx23 was increased by growing in medium at low (4.0) pH and decreased by growing at high (7.0) pH. The cytosolic pH in the leaves of the mutant was significantly higher than that in the wild type. chx23 mutants displayed a high sensitivity to NaCl. Together, these data indicate that CHX23 is a probable chloroplast Na(+)(K(+))/H(+) exchanger important for pH homeostasis and chloroplast development and function.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

