DNA display II. Genetic manipulation of combinatorial chemistry libraries for small-molecule evolution
- PMID: 15221028
- PMCID: PMC434149
- DOI: 10.1371/journal.pbio.0020174
DNA display II. Genetic manipulation of combinatorial chemistry libraries for small-molecule evolution
Abstract
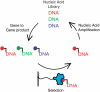
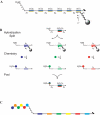
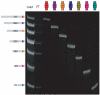
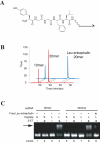
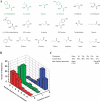
Biological in vitro selection techniques, such as RNA aptamer methods and mRNA display, have proven to be powerful approaches for engineering molecules with novel functions. These techniques are based on iterative amplification of biopolymer libraries, interposed by selection for a desired functional property. Rare, promising compounds are enriched over multiple generations of a constantly replicating molecular population, and subsequently identified. The restriction of such methods to DNA, RNA, and polypeptides precludes their use for small-molecule discovery. To overcome this limitation, we have directed the synthesis of combinatorial chemistry libraries with DNA "genes," making possible iterative amplification of a nonbiological molecular species. By differential hybridization during the course of a traditional split-and-pool combinatorial synthesis, the DNA sequence of each gene is read out and translated into a unique small-molecule structure. This "chemical translation" provides practical access to synthetic compound populations 1 million-fold more complex than state-of-the-art combinatorial libraries. We carried out an in vitro selection experiment (iterated chemical translation, selection, and amplification) on a library of 10(6) nonnatural peptides. The library converged over three generations to a high-affinity protein ligand. The ability to genetically encode diverse classes of synthetic transformations enables the in vitro selection and potential evolution of an essentially limitless collection of compound families, opening new avenues to drug discovery, catalyst design, and the development of a materials science "biology."
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures

References
-
- Anderson GW, Zimmerman JE, Callahan FM. N-hydroxysuccinimide esters in peptide synthesis. J Am Chem Soc. 1963;85:3039. - PubMed
-
- Barrett RW, Cwirla SE, Ackerman MS, Olson AM, Peters EA, et al. Selective enrichment and characterization of high-affinity ligands from collections of random peptides on filamentous phage. Anal Biochem. 1992;204:357–364. - PubMed
-
- Bittker JA, Phillips KJ, Liu DR. Recent advances in the in vitro evolution of nucleic acids. Curr Opin Chem Biol. 2002;6:367–374. - PubMed
-
- Breinbauer R, Vetter IR, Waldmann H. From protein domains to drug candidates: Natural products as guiding principles in the design and synthesis of compound libraries. Angew Chem Int Ed Engl. 2002;41:2879–2890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

