Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum
- PMID: 15225124
- PMCID: PMC1134069
- DOI: 10.1042/BJ20040742
Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum
Abstract
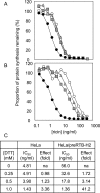
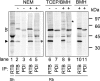
Cells expressing ricin B chain within the secretory pathway are significantly more resistant to intoxication by ricin holotoxin but not to other cytotoxins that exploit similar endocytic routes to the cytosol. Furthermore, cells expressing the related B chain of abrin are protected against both incoming abrin and ricin. These phenotypes can be correlated with the abilities of the respective B chains to form disulphide-linked A-B holotoxins, since abrin B chain forms heterodimers with either abrin or ricin A chains, whereas ricin B chain forms heterodimers with ricin A chain only. In the ricin B-expressing cells, this newly made lectin disappears with biphasic kinetics comprising a retention phase followed by slow turnover and disposal after disengagement from calnexin cycle components. Interference with ricin cytotoxicity occurs during the early retention phase when ricin B chain is associated with PDI (protein disulphide-isomerase). The data show that retrotranslocation of incoming toxin is impeded by PDI-catalysed formation of heterodimers between endogenous B and A chains derived from reduced holotoxin, thus proving that reduction of ricin occurs in the endoplasmic reticulum. In contrast with other toxins, ricin does not appear to require either proteolytic cleavage or unfolding for PDI-catalysed reduction.
Figures
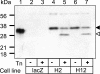
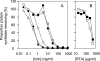


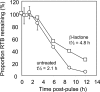
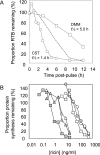

References
-
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987;262:8128–8130. - PubMed
-
- Olsnes S., Pihl A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry. 1973;12:3121–3126. - PubMed
-
- Lord J. M., Jolliffe N. A., Marsden C. J., Pateman S. C., Smith D. C., Spooner R. A., Watson P. D., Roberts L. M. Ricin: mechanisms of cytotoxicity. Toxicol. Rev. 2003;22:53–64. - PubMed
-
- Yoshida T., Chen C. C., Zhang M. S., Wu H. C. Disruption of the Golgi apparatus by brefeldin A inhibits the cytotoxicity of ricin, modeccin, and Pseudomonas toxin. Exp. Cell Res. 1991;192:389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

