Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy: a prospective single-centre study
- PMID: 15225368
- PMCID: PMC464872
- DOI: 10.1186/ar1182
Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy: a prospective single-centre study
Abstract
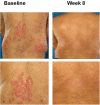
Psoriasis and psoriatic arthritis are inflammatory diseases that respond well to anti-tumour necrosis factor-alpha therapy. To evaluate the effects of anti-tumour necrosis factor-alpha treatment on expression of adhesion molecules and angiogenesis in psoriatic lesional skin and synovial tissue, we performed a prospective single-centre study with infliximab therapy combined with stable methotrexate therapy. Eleven patients with both active psoriasis and psoriatic arthritis received infusions of infliximab (3 mg/kg) at baseline, and at weeks 2, 6, 14 and 22 in an open-label study. In addition, patients continued to receive stable methotrexate therapy in dosages ranging from 5 to 20 mg/week. Clinical assessments, including Psoriasis Area and Severity Index (PASI) and Disease Activity Score (DAS), were performed at baseline and every 2 weeks afterward. In addition, skin biopsies from a target psoriatic plaque and synovial tissue biopsies from a target joint were taken before treatment and at week 4. Immunohistochemical analysis was performed to detect the number of blood vessels, the expression of adhesion molecules and the presence of vascular growth factors. Stained sections were evaluated by digital image analysis. At week 16, the mean PASI was reduced from 12.3 +/- 2.4 at baseline to 1.8 +/- 0.4 (P <or= 0.02). The mean DAS was reduced from 6.0 +/- 0.5 to 3.6 +/- 0.6 (P <or= 0.02). We found some fluctuations in DAS response as compared with the change in PASI, with the latter exhibiting a steady decrease over time. After 4 weeks the cell infiltrate was reduced in both skin and synovium. There was a significant reduction in the number of blood vessels in dermis and synovium at week 4. A significant reduction in the expression of alphavbeta3 integrin, a marker of neovascularization, was also found in both skin and synovium at week 4. In addition, a significant reduction in the expression of adhesion molecules was observed in both skin and synovium at week 4. We also observed a trend toward reduced expression of vascular endothelial growth factor in both skin and synovium. In conclusion, low-dose infliximab treatment leads to decreased neoangiogenesis and deactivation of the endothelium, resulting in decreased cell infiltration and clinical improvement in psoriasis and psoriatic arthritis.
Figures
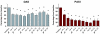

References
-
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. - DOI - PubMed
-
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P, ATTRACT Study Group Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet. 1999;354:1932–1939. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
-
- Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

