A new family of potent AB(5) cytotoxins produced by Shiga toxigenic Escherichia coli
- PMID: 15226357
- PMCID: PMC2213318
- DOI: 10.1084/jem.20040392
A new family of potent AB(5) cytotoxins produced by Shiga toxigenic Escherichia coli
Erratum in
- J Exp Med. 2004 Dec 6;200(11):1525
Abstract
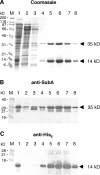
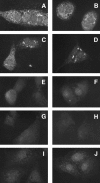
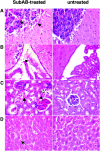
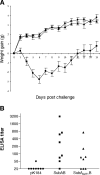
The Shiga toxigenic Escherichia coli (STEC) O113:H21 strain 98NK2, which was responsible for an outbreak of hemolytic uremic syndrome, secretes a highly potent and lethal subtilase cytotoxin that is unrelated to any bacterial toxin described to date. It is the prototype of a new family of AB(5) toxins, comprising a single 35-kilodalton (kD) A subunit and a pentamer of 13-kD B subunits. The A subunit is a subtilase-like serine protease distantly related to the BA_2875 gene product of Bacillus anthracis. The B subunit is related to a putative exported protein from Yersinia pestis, and binds to a mimic of the ganglioside GM2. Subtilase cytotoxin is encoded by two closely linked, cotranscribed genes (subA and subB), which, in strain 98NK2, are located on a large, conjugative virulence plasmid. Homologues of the genes are present in 32 out of 68 other STEC strains tested. Intraperitoneal injection of purified subtilase cytotoxin was fatal for mice and resulted in extensive microvascular thrombosis, as well as necrosis in the brain, kidneys, and liver. Oral challenge of mice with E. coli K-12-expressing cloned subA and subB resulted in dramatic weight loss. These findings suggest that the toxin may contribute to the pathogenesis of human disease.
Figures
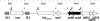



References
-
- Fan, E., E.A. Merritt, C.L.M.J. Verlinde, and W.G.J. Hol. 2000. AB(5) toxins: structures and inhibitor design. Curr. Opin. Struct. Biol. 10:680–686. - PubMed
-
- Schmidt, H., J. Scheef, H.I. Huppertz, M. Frosch, and H. Karch. 1999. Escherichia coli O157:H7 and O157:H(-) strains that do not produce Shiga toxin: phenotypic and genetic characterization of isolates associated with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3491–3496. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

