Gramicidin-perforated patch recording revealed the oscillatory nature of secretory Cl- movements in salivary acinar cells
- PMID: 15226364
- PMCID: PMC2229610
- DOI: 10.1085/jgp.200308948
Gramicidin-perforated patch recording revealed the oscillatory nature of secretory Cl- movements in salivary acinar cells
Abstract
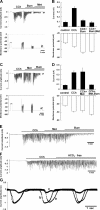
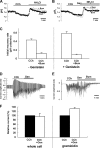
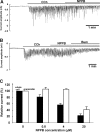
Elevations of cytoplasmic free calcium concentrations ([Ca(2+)](i)) evoked by cholinergic agonists stimulate isotonic fluid secretion in salivary acinar cells. This process is driven by the apical exit of Cl(-) through Ca(2+)-activated Cl(-) channels, while Cl(-) enters the cytoplasm against its electrochemical gradient via a loop diuretic-sensitive Na(+)-K(+)-2Cl(-) cotransporter (NKCC) and/or parallel operations of Cl(-)-HCO(3)(-) and Na(+)-H(+) exchangers, located in the basolateral membrane. To characterize the contributions of those activities to net Cl(-) secretion, we analyzed carbachol (CCh)-activated Cl(-) currents in submandibular acinar cells using the "gramicidin-perforated patch recording configuration." Since the linear polypeptide antibiotic gramicidin creates monovalent cation-selective pores, CCh-activated Cl(-) currents in the gramicidin-perforated patch recording were carried by Cl(-) efflux via Cl(-) channels, dependent upon Cl(-) entry through Cl(-) transporters expressed in the acinar cells. CCh-evoked oscillatory Cl(-) currents were associated with oscillations of membrane potential. Bumetanide, a loop diuretic, decreased the CCh-activated Cl(-) currents and hyperpolarized the membrane potential. In contrast, neither methazolamide, a carbonic anhydrase inhibitor, nor elimination of external HCO(3)(-) had significant effects, suggesting that the cotransporter rather than parallel operations of Cl(-)-HCO(3)(-) and Na(+)-H(+) exchangers is the primary Cl(-) uptake pathway. Pharmacological manipulation of the activities of the Ca(2+)-activated Cl(-) channel and the NKCC revealed that the NKCC plays a substantial role in determining the amplitude of oscillatory Cl(-) currents, while adjusting to the rate imposed by the Ca(2+)-activated Cl(-) channel, in the gramicidin-perforated patch configuration. By concerting with and being controlled by the cation steps, the oscillatory form of secretory Cl(-) movements may effectively provide a driving force for fluid secretion in intact acinar cells.
Figures


Similar articles
-
Involvement of sodium-glucose cotransporter-1 activities in maintaining oscillatory Cl- currents from mouse submandibular acinar cells.J Comp Physiol B. 2024 Feb;194(1):21-32. doi: 10.1007/s00360-024-01532-w. Epub 2024 Feb 3. J Comp Physiol B. 2024. PMID: 38308715 Free PMC article.
-
Suppression of carbachol-induced oscillatory Cl- secretion by forskolin in rat parotid and submandibular acinar cells.Am J Physiol Gastrointest Liver Physiol. 2008 Mar;294(3):G738-47. doi: 10.1152/ajpgi.00239.2007. Epub 2008 Jan 10. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18187520
-
Chloride secretion by porcine ciliary epithelium: New insight into species similarities and differences in aqueous humor formation.Invest Ophthalmol Vis Sci. 2006 Dec;47(12):5428-36. doi: 10.1167/iovs.06-0180. Invest Ophthalmol Vis Sci. 2006. PMID: 17122133
-
Isosmotic modulation of cell volume and intracellular ion activities during stimulation of single exocrine cells.J Exp Zool. 1994 Feb 1;268(2):104-10. doi: 10.1002/jez.1402680206. J Exp Zool. 1994. PMID: 8301250 Review.
-
Mechanisms of CSF secretion by the choroid plexus.Microsc Res Tech. 2001 Jan 1;52(1):49-59. doi: 10.1002/1097-0029(20010101)52:1<49::AID-JEMT7>3.0.CO;2-C. Microsc Res Tech. 2001. PMID: 11135448 Review.
Cited by
-
Non-steroidal anti-inflammatory drugs increase insulin release from beta cells by inhibiting ATP-sensitive potassium channels.Br J Pharmacol. 2007 Jun;151(4):483-93. doi: 10.1038/sj.bjp.0707259. Epub 2007 Apr 16. Br J Pharmacol. 2007. PMID: 17435793 Free PMC article.
-
Long-term dexamethasone treatment diminishes store-operated Ca2+ entry in salivary acinar cells.Int J Oral Sci. 2019 Jan 3;11(1):1. doi: 10.1038/s41368-018-0031-0. Int J Oral Sci. 2019. PMID: 30602784 Free PMC article.
-
GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 β-cells.PLoS One. 2011;6(10):e26225. doi: 10.1371/journal.pone.0026225. Epub 2011 Oct 21. PLoS One. 2011. PMID: 22031825 Free PMC article.
-
Involvement of sodium-glucose cotransporter-1 activities in maintaining oscillatory Cl- currents from mouse submandibular acinar cells.J Comp Physiol B. 2024 Feb;194(1):21-32. doi: 10.1007/s00360-024-01532-w. Epub 2024 Feb 3. J Comp Physiol B. 2024. PMID: 38308715 Free PMC article.
-
Frequency spectrum of transepithelial potential difference reveals transport-related oscillations.Biophys J. 2009 Sep 16;97(6):1530-7. doi: 10.1016/j.bpj.2009.05.063. Biophys J. 2009. PMID: 19751657 Free PMC article.
References
-
- Allen, T.W., O.S. Andersen, and B. Roux. 2003. Structure of gramicidin A in a lipid bilayer environment determined using molecular dynamics simulations and solid-state NMR data. J. Am. Chem. Soc. 125:9868–9877. - PubMed
-
- Andersen, O.S., and R.E. Koeppe. 1992. Molecular determinants of channel function. Physiol. Rev. 72:S89–S158. - PubMed
-
- Dowd, B.F., and B. Forbush. 2003. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J. Biol. Chem. 278:27347–27353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

