The bioactive Taxol conformation on beta-tubulin: experimental evidence from highly active constrained analogs
- PMID: 15226503
- PMCID: PMC454156
- DOI: 10.1073/pnas.0403459101
The bioactive Taxol conformation on beta-tubulin: experimental evidence from highly active constrained analogs
Abstract
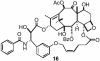
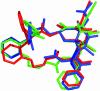
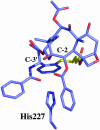
The important anticancer drug Taxol (paclitaxel) binds to tubulin in a stoichiometric ratio and promotes its assembly into microtubules. The conformation of microtubule-bound drug has been the subject of intense study, and various suggestions have been made. In this work we present experimental and theoretical evidence that Taxol adopts a T-shaped conformation when it is bound to tubulin.
Figures
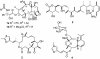
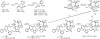
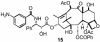

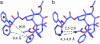
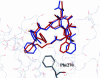
References
-
- Crown, J. & O'Leary, M. (2000) Lancet 355, 1176-1178. - PubMed
-
- Nabholtz, J.-M., Tonkin, K., Smylie, M., Au, H.-J., Lindsay, M.-A. & Mackey, J. (2000) Exp. Opin. Pharmacother. 1, 187-206. - PubMed
-
- Miller, K. D. & Sledge, G. W., Jr. (1999) Cancer Invest. 17, 121-136. - PubMed
-
- Schiff, P. B., Fant, J. & Horwitz, S. B. (1979) Nature 277, 665-667. - PubMed
-
- Ringel, I. & Horwitz, S. B. (1991) J. Natl. Cancer Inst. 83, 288-291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

