Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer
- PMID: 15226765
- PMCID: PMC2364779
- DOI: 10.1038/sj.bjc.6602001
Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer
Abstract
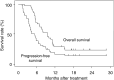
Gemcitabine has been reported to be a potent radiosensitiser in human pancreatic cell lines. This study was conducted to evaluate the efficacy and toxicity of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. In all, 42 patients with pancreatic cancer that was unresectable but confined to the pancreatic region were treated with external-beam radiation (50.4 Gy in 28 fractions over 5.5 weeks) and weekly gemcitabine (250 mg m(-2), 30-min infusion). Maintenance gemcitabine (1000 mg m(-2) weekly x 3 every 4 weeks) was initiated 1 month after the completion of the chemoradiotherapy and continued until disease progression or unacceptable toxicity. Of the 42 patients, 38 (90%) completed the scheduled course of chemoradiotherapy. The major toxicity was leucopenia and anorexia. There was one death attributed to duodenal bleeding and sepsis. The median survival time was 9.5 months and the 1-year survival rate was 28%. The median progression-free survival time was 4.4 months. In 35 patients with documented disease progression at the time of analysis, 34 (97%) showed distant metastasis as the cause of the initial disease progression. The chemoradiotherapy used in this study has a moderate activity against locally advanced pancreatic cancer and an acceptable toxicity profile. Future investigations for treatment with more systemic effects are warranted.
Figures
Similar articles
-
Phase II study of radiation therapy combined with weekly low-dose gemcitabine for locally advanced, unresectable pancreatic cancer.Am J Clin Oncol. 2011 Apr;34(2):115-9. doi: 10.1097/COC.0b013e3181c4c7a8. Am J Clin Oncol. 2011. PMID: 20065850 Clinical Trial.
-
Chemoradiotherapy with twice-weekly administration of low-dose gemcitabine for locally advanced pancreatic cancer.World J Gastroenterol. 2008 Sep 14;14(34):5311-5. doi: 10.3748/wjg.14.5311. World J Gastroenterol. 2008. PMID: 18785284 Free PMC article. Clinical Trial.
-
Concomitant chemoradiotherapy with low-dose weekly gemcitabine for nonmetastatic unresectable pancreatic cancer.Turk J Gastroenterol. 2011 Feb;22(1):60-4. doi: 10.4318/tjg.2011.0158. Turk J Gastroenterol. 2011. PMID: 21480113
-
New applications of gemcitabine and future directions in the management of pancreatic cancer.Cancer. 2002 Aug 15;95(4 Suppl):941-5. doi: 10.1002/cncr.10753. Cancer. 2002. PMID: 12209675 Review.
-
On the development of gemcitabine-based chemoradiotherapy regimens in pancreatic cancer.Cancer. 2002 Aug 15;95(4 Suppl):933-40. doi: 10.1002/cncr.10754. Cancer. 2002. PMID: 12209674 Review.
Cited by
-
The technical feasibility of an image-guided intensity-modulated radiotherapy (IG-IMRT) to perform a hypofractionated schedule in terms of toxicity and local control for patients with locally advanced or recurrent pancreatic cancer.Radiat Oncol. 2012 Dec 5;7:203. doi: 10.1186/1748-717X-7-203. Radiat Oncol. 2012. PMID: 23216796 Free PMC article.
-
Phase-I trial of oral fluoropyrimidine anticancer agent (S-1) with concurrent radiotherapy in patients with unresectable pancreatic cancer.Br J Cancer. 2007 May 7;96(9):1353-7. doi: 10.1038/sj.bjc.6603735. Epub 2007 Apr 17. Br J Cancer. 2007. PMID: 17437021 Free PMC article. Clinical Trial.
-
Gemcitabine plus nab-paclitaxel for locally advanced or borderline resectable pancreatic cancer.Sci Rep. 2019 Nov 7;9(1):16187. doi: 10.1038/s41598-019-52486-x. Sci Rep. 2019. PMID: 31700023 Free PMC article. Clinical Trial.
-
Prognostic implication of para-aortic lymph node metastasis in resectable pancreatic cancer.World J Surg. 2007 Jan;31(1):147-54. doi: 10.1007/s00268-005-0730-5. World J Surg. 2007. PMID: 17171496
-
Radiation therapy and photodynamic therapy for biliary tract and ampullary carcinomas.J Hepatobiliary Pancreat Surg. 2008;15(1):63-8. doi: 10.1007/s00534-007-1281-y. Epub 2008 Feb 16. J Hepatobiliary Pancreat Surg. 2008. PMID: 18274845 Free PMC article.
References
-
- Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413 - PubMed
-
- Chakravarthy A, Abrams RA (1997) Radiation therapy and 5-fluorouracil in pancreatic cancer. Semin Radiat Oncol 7: 291–299 - PubMed
-
- Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters PW, Lee JE, Lenzi R, Vauthey JN, Wong AB, Phan T, Nguyen Q, Janjan NA (2002) Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys 52: 1293–1302 - PubMed
-
- Gastrointestinal Tumor Study Group (1981) Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads+5-fluorouracil), and high dose radiation+5-fluorouracil. Cancer 48: 1705–1710 - PubMed
-
- Gastrointestinal Tumor Study Group (1988) Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 80: 751–755 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical