Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials
- PMID: 15229247
- PMCID: PMC6729228
- DOI: 10.1523/JNEUROSCI.4848-03.2004
Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials
Abstract
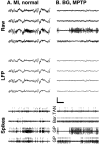
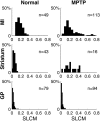
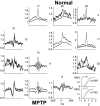
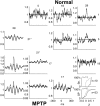
Cortical local field potentials (LFPs) reflect synaptic potentials and accordingly correlate with neuronal discharge. Because LFPs are coherent across substantial cortical areas, we hypothesized that cortical spike correlations could be predicted from them. Because LFPs recorded in the basal ganglia (BG) are also correlated with neuronal discharge and are clinically accessible in Parkinson's disease patients, we were interested in testing this hypothesis in the BG, as well. We recorded LFPs and unit discharge from multiple electrodes, which were placed in primary motor cortex or in the basal ganglia (striatum and pallidum) of two monkeys before and after rendering them parkinsonian with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. We used the method of partial spectra to construct LFP predictors of the spike cross-correlation functions (CCFs). The predicted CCF is an estimate of the correlation between two neurons under the assumption that their association is explained solely by the association of each with the LFP recorded on a third electrode. In the normal condition, the predictors account for cortical rate covariations but not for the association among the tonically active neurons of the striatum. In the parkinsonian condition, with the appearance of 10 Hz oscillations throughout the cortex-basal ganglia networks, the LFP predictors account remarkably better for the CCFs in both the cortex and the basal ganglia. We propose that, in the parkinsonian condition, the cortex-basal ganglia networks are more tightly related to global modes of brain dynamics that are echoed in the LFP.
Figures

References
-
- Abeles M (1974) A journey into the brain. In: Signal analysis and pattern recognition in biomedical engineering (Inbar GF, ed), pp 41-59. New York: Wiley.
-
- Abeles M (1982) Local cortical circuits. (Braitenberg V, Barlow HB, Bullock H, Florey E, Grüsser O-J, Peters A, eds). New York: Springer.
-
- Aosaki T, Kimura M, Graybiel AM (1995) Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol 73: 1234-1252. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A (1996) Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868-1871. - PubMed
-
- Asanuma H (1989) The motor cortex. New York: Raven.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
